1. Background
The incidence of depression has surged dramatically during the COVID-19 pandemic (1). Energy metabolism plays a pivotal role in the physiological functions of the nervous system, including neuroplasticity, neurotransmitter release, and neural differentiation. Disorders in energy metabolism are among the primary causes of neuropsychiatric diseases (1). Glucose serves as the main energy source for the brain through mitochondrial phosphorylation (2). Furthermore, astrocytes play crucial roles in brain metabolism and neurotransmitter homeostasis (3). Astrocyte activity is reflected in the upregulation or downregulation of glial fibrillary acidic protein (GFAP), which is primarily expressed in astrocytes and detectable in cerebrospinal fluid (CSF) and bloodstream (3). Its expression fluctuates in psychological disorders (4).
SARS-CoV-2, the virus responsible for COVID-19, has a high affinity for neurons and glia in the central nervous system (CNS) through binding to the angiotensin-converting enzyme 2 (ACE2) receptor (5). The virus can lead to neuroinflammation, adaptive immunity, and autoimmunity, affecting various neurological and psychiatric aspects of COVID-19 (5). Astrocytes and microglia maintain homeostasis at different biological levels by transporting ions, uptaking neurotransmitters, scavenging reactive oxygen species, and supporting glycogen synthesis and storage (6, 7).
Lactate utilization is emerging as a novel regulatory link in brain metabolism. Lactate dehydrogenase (LDH) catalyzes the conversion of lactate to pyruvate and facilitates glycolysis (8). Dysregulation or decreased LDH activity may lead to lactate accumulation, as observed in neurodegenerative disorders and post-stroke depression (9, 10). While substantial evidence focuses on decreased LDH levels in depressive patients, the association between lactate metabolism and depression remains elusive. Additionally, creatine, a basic energy source for cellular physiology, plays a critical role in cellular metabolism via ATP replenishment. Creatine kinase (CK), discovered in muscle fibers in 1927, is vital in maintaining cellular energy homeostasis by restoring ATP reserves (11-13). Creatine kinase is present in the cytosol and mitochondria of various cells and is released into the blood circulation through the lymphatic system under resting conditions (14-16). Elevated CK levels are commonly observed in COVID-19 patients (17).
2. Objectives
However, the relationship between serum levels of GFAP, CK, and LDH and the development of depression in COVID-19 patients remains unclear. Thus, this study aims to investigate the potential association between astrocyte activation, serum CK and LDH levels, and the severity of depressed mood in individuals diagnosed with COVID-19.
3. Methods
3.1. Subjects
In this cross-sectional study, a total of 150 patients with confirmed COVID-19 infection (75 males and 75 females) were examined. Venous blood samples (6 mL) were collected from all participants in a fasting state (between 6:30 am and 9:00 am) within the first 24 hours after admission. Various biochemical factors were measured in the blood samples. All subjects had experienced symptoms for 13 - 16 weeks following their initial COVID-19 infection and were referred to Imam Khomeini Hospital in Ilam, western Iran, during 2022 and 2023. The subjects were matched for both sex and age to ensure comparability across the groups.
3.2. Inclusion Criteria
The study included COVID-19 patients aged between 32 and 42 years. All subjects exhibited symptoms such as fatigue, shortness of breath, and neurological and cognitive dysfunction, which significantly impacted their daily lives. These symptoms persisted for at least two months and could not be attributed to any other diagnosis. The onset of this condition occurred approximately three months after the subjects tested positive for SARS-CoV-2.
3.3. Exclusion Criteria
Subjects with any metabolic diseases known to affect CK and LDH levels or with a history of head trauma prior to admission were excluded from the study.
3.4. Glial Fibrillary Acidic Protein Quantification
GFAP levels were measured in the serum of all subjects using a Human GFAP ELISA Kit, following the manufacturer's protocol. The detection range of the assay was 15.63 - 1000 pg/mL. After the enzyme reaction produced a yellowish color, a stop solution was added to terminate the reaction, and the optical density was measured spectrophotometrically at 450 nm.
3.5. Creatine Kinase Quantification
Serum CK levels were measured using a standard ELISA technique with a CK-NAC (CPK) quantification kit from Biorex Fars, following the manufacturer's protocol. One volume of R2 reagent was mixed with five volumes of R1 reagent. Then, 40 µL of the samples was added to 1000 µL of the working reagent, and the absorbance was read spectrophotometrically at 340 nm after two minutes. The kit's minimum detectable activity was 2 U/L. Normal CK levels range from 55 - 170 U/L in males and 30 - 135 U/L in females.
3.6. Lactate Dehydrogenase Quantification
Serum LDH levels were measured using the LDH assay kit from Biomed, following the manufacturer's instructions. Four units of R1 reagent were mixed with one unit of R2 reagent, and the final solution was measured photometrically at 340 nm. The kit's minimum measurable sensitivity was 40 U/L, with a maximum detection level of 1200 U/L. The normal reference range for LDH is 140 - 280 U/L for both males and females.
3.7. Beck Depression Inventory-II
Depressive status in patients was assessed using the Beck Depression Inventory-II (BDI-II), a 21-item questionnaire (18). The scoring interpretation is as follows: A score of 0 - 13 indicates no depression, 14 - 19 reflects mild depression, 20 - 28 corresponds to moderate depression, and 29 - 63 represents severe depression.
3.8. Statistical Analysis
Data analysis was conducted using Prism GraphPad software. A significance level of P < 0.05 was set with a 95% confidence interval. The Kolmogorov-Smirnov test and Shapiro-Wilk test were applied to assess the normality of data distribution. Spearman non-parametric tests were utilized to evaluate the correlation between variables. Data are consistently presented as mean ± SEM throughout the study.
4. Results
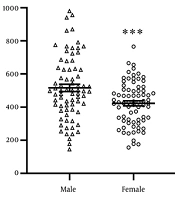
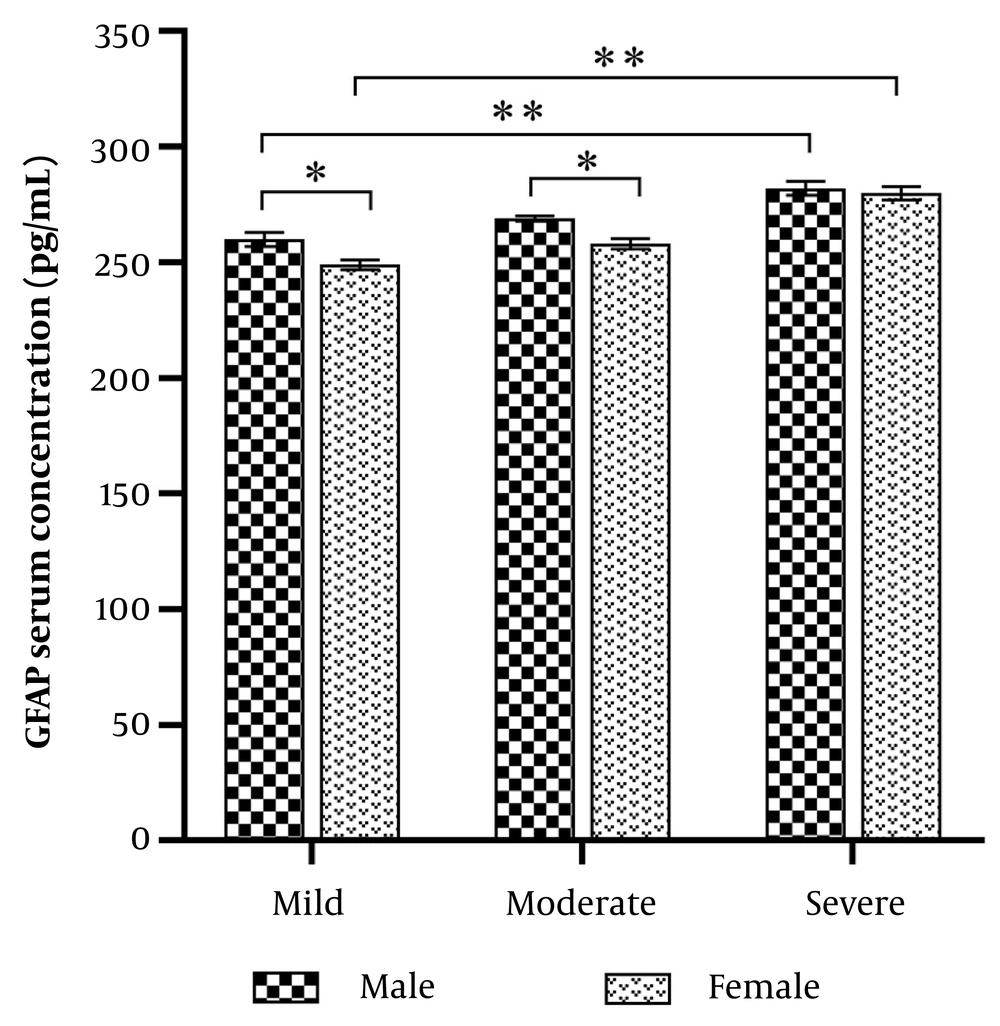
The mean age of the participants was 36.6 ± 0.67 years in males and 35 ± 0.76 years in females. Serum GFAP levels were measured in all subjects. In males, the mean GFAP levels were 260 ± 3 pg/mL for mild depression, 269 ± 1.2 pg/mL for moderate depression, and 282 ± 3 pg/mL for severe depression. In females, GFAP levels were 249 ± 2.1 pg/mL for mild depression, 258 ± 2.2 pg/mL for moderate depression, and 280 ± 2.9 pg/mL for severe depression. GFAP levels were significantly higher in severe depression compared to mild depression (Figure 1 P = 0.001). Additionally, GFAP levels were significantly higher in males than females with mild and moderate depression (P < 0.001).
Glial fibrillary acidic protein (GFAP) serum levels in males and females with COVID-19 infection and depressive disorders. Glial fibrillary acidic protein levels were increased significantly in male patients vs. females (* P < 0.01). Glial fibrillary acidic protein serum levels were significantly increased in severe depression compared with the mild status in both males and females (** P = 0.001).
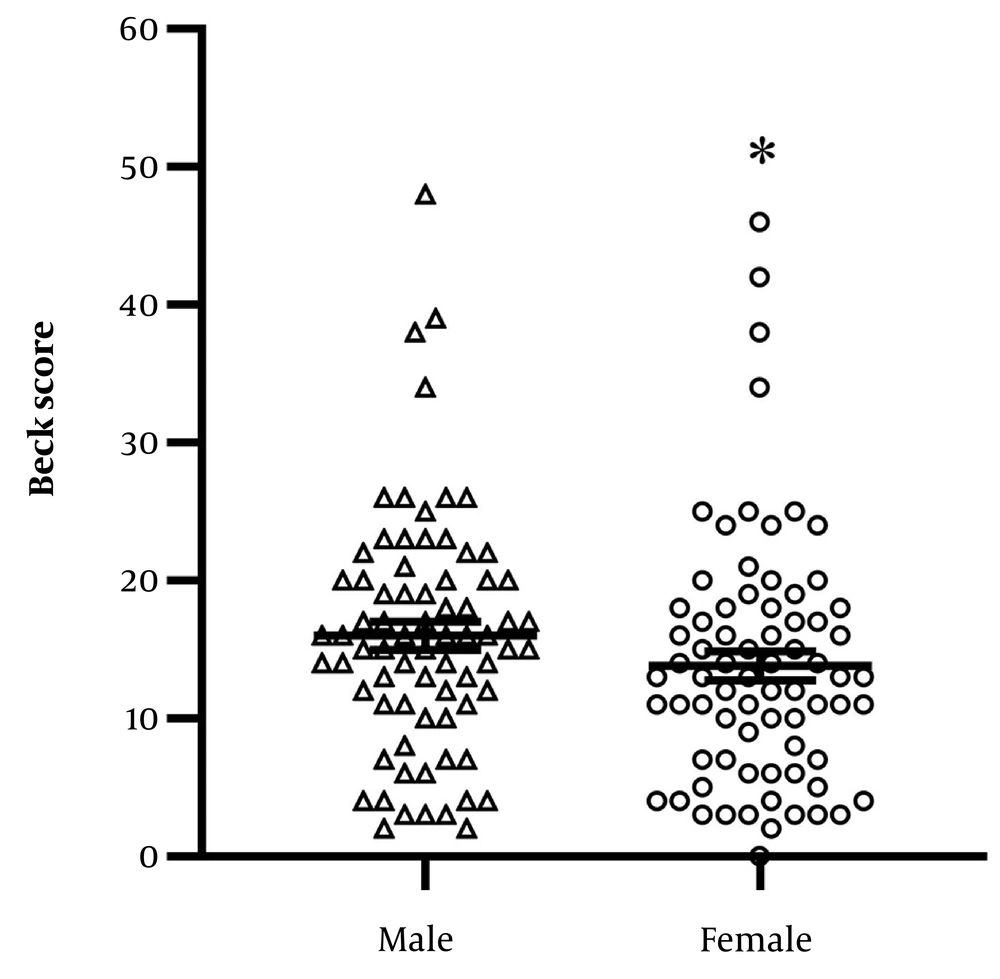
The BDI-II scores were 16.15 ± 1 in males and 13.67 ± 1.05 in females, with significantly higher scores observed in males compared to females (Figure 2 P = 0.016). No significant correlation was found between Beck scores and the age of participants (r = -0.11, 95% CI = -0.2704 to 0.555).
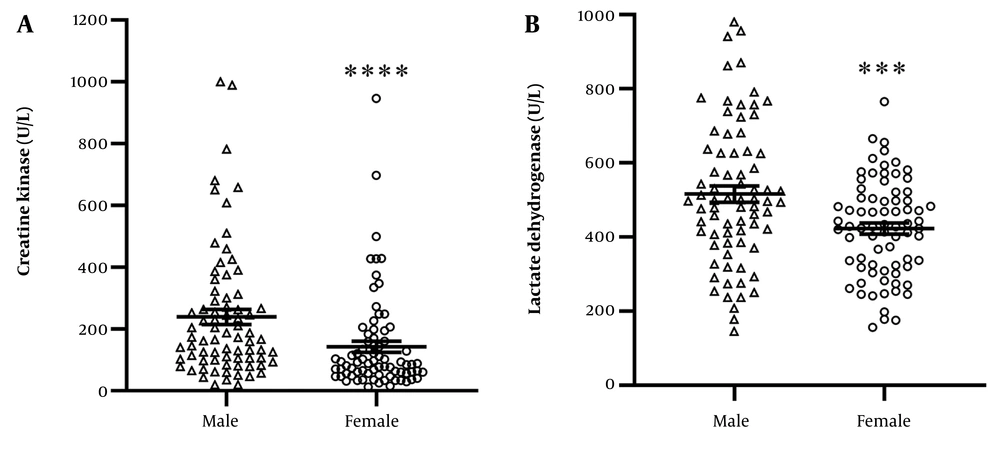
The mean serum CK levels in COVID-19 patients are shown in Figure 3A. CK levels were 142 ± 18.08 U/L in females and significantly higher at 239 ± 24.05 U/L in males (P < 0.0001). Serum LDH levels were 423.6 ± 14.99 U/L in females and significantly increased to 516 ± 22.01 U/L in males (P = 0.0006, Figure 3B).
A, the mean ± SEM of serum level of creatine kinase (CK) in males and females infected with COVID-19 is shown. The level of CK is significantly higher in males vs. females (**** P < 0.0001); B, the mean ± SEM of serum level of lactate dehydrogenase (LDH) in males and females infected with COVID-19 is shown. The level of LDH is significantly higher in males vs. females (*** P = 0.0006).
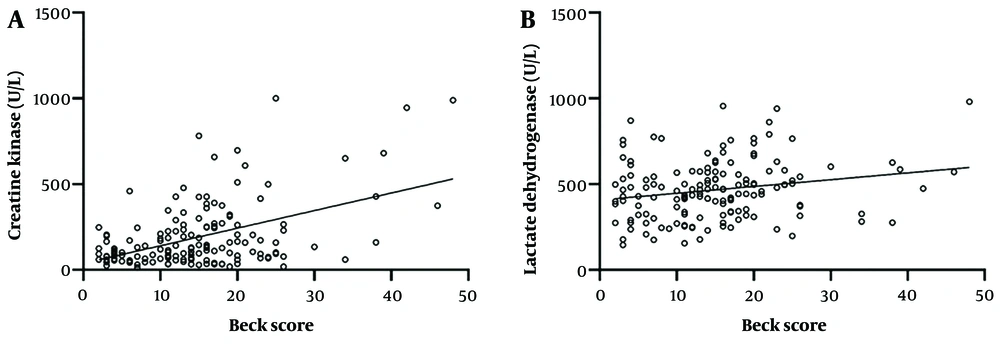
Furthermore, there was a significant positive correlation between CK levels and Beck scores (r = 0.33, 95% CI = 0.176 to 0.469, Figure 4A). Similarly, there was a significant positive correlation between LDH serum levels and Beck scores in COVID-19 patients (r = 0.22, 95% CI = 0.042 to 0.358, Figure 4B), indicating a strong relationship between depression severity and serum CK and LDH levels.
A, the correlation between creatine kinase (CK) and beck scores is shown. There is positive correlation between CK serum levels and beck scores (r = 0.33, 95% CI = 0.176 to 0.469); B, the correlation between lactate dehydrogenase (LDH) and beck scores is shown. There is a positive correlation between CK serum levels and beck scores (r = 0.22, 95% CI = 0.042 to 0.358).
5. Discussion
This section outlines the observed correlation between depression severity, as measured by Beck scores, and elevated serum levels of GFAP, CK, and LDH in individuals infected with COVID-19. The findings suggest that as depressive symptoms intensify in COVID-19 patients, there is a corresponding increase in these biomarkers. Notably, the study found that depressive disorders were more prevalent among male COVID-19 patients compared to females.
It is estimated that approximately 10 - 20% of individuals infected with SARS-CoV-2 develop fluctuating symptoms that persist beyond 12 weeks after the acute infection, a condition known as long COVID syndrome (19). Previous literature has linked COVID-19 infection with various psychiatric conditions, including depression, anxiety, and insomnia, with depression being more prevalent than anxiety and stress in these patients (20, 21).
Glial fibrillary acidic protein, a major intermediate filament protein in glial cells, is predominantly expressed in astrocytes and plays crucial roles in regeneration, synaptic plasticity, and reactive gliosis within the CNS (22). It is considered as an inflammatory blood biomarker associated with various brain insults, including neurodegenerative diseases (23). The inflammatory response can affect microglial activity, and astrocyte activation has the potential to disrupt neurotransmitter balance, thereby exacerbating depressive symptoms (24). A study by Ellis et al. demonstrated elevated GFAP levels in CSF in patients experiencing depressed mood during HIV infection, reflecting greater astrocyte activation (24). In our study, we observed a more pronounced increase in GFAP levels associated with depressive mood in males compared to females infected with COVID-19. The metabolic support of neurons is closely related to astrocyte function (25). While some studies have reported an inverse correlation between GFAP levels and neurological complaints, elevated GFAP levels (>700 pg/mL) have been observed in unipolar depression, possibly due to the analysis of GFAP in CSF samples as opposed to blood samples, as was done in this study (26, 27). Astrocytic abnormalities, primarily found in cortical and subcortical networks associated with mood disorders, may contribute to these findings (4).
In another study by Ellis et al. in 2022, males exhibited higher GFAP levels than females, which supports our findings in the current study.
Similarly, elevated CK levels are common in COVID-19 patients, with levels increasing as disease severity worsens (28). Additionally, LDH levels are significantly altered in SARS-CoV-2-infected patients and may serve as a biomarker for the severity of COVID-19 (29). Both CK and LDH play critical roles in brain energy metabolism, with CK facilitating the transfer of high-energy phosphate from ATP to creatine, thereby maintaining ATP homeostasis, and LDH catalyzing the conversion of pyruvate to lactate in anaerobic glucose metabolism (1, 30, 31).
The results of this study align with previous findings demonstrating an association between elevated CK and LDH serum levels and the incidence of depression in COVID-19 patients. Creatine kinase is present not only in neurons but also in glial cells such as astrocytes and oligodendrocytes within the CNS (32). In 1990, Balaita et al. demonstrated increased serum CK levels in individuals with depressive disorders, regardless of whether they had unipolar or bipolar depression (32). Additionally, electroconvulsive therapy (ECT), used for drug-resistant depression, has been shown to reduce CK activity in various brain regions (33).
Associations between serum CK and LDH levels and different states of depression have been indicated in some studies (34). Furthermore, previous research found higher levels of CK and LDH in manic patients (35), while other studies revealed lower serum CK levels (34) in stressful environments and lower LDH levels in depressed patients (1, 34).
COVID-19 can cause hypoxia through direct lung injury, leading to pneumonia and ARDS, which impair gas exchange due to alveolar inflammation and fluid accumulation. "Silent hypoxia" is another phenomenon where patients have low oxygen levels without typical symptoms, possibly due to impaired chemoreception. Vascular complications such as pulmonary thromboembolism and endothelial dysfunction also contribute by obstructing blood flow and impairing oxygen delivery to tissues (36, 37).
Hypoxia in COVID-19 patients can lead to severe consequences, including organ dysfunction and an increased risk of mortality. Prolonged oxygen deficiency affects essential organs such as the brain, kidneys, and heart, potentially resulting in multi-organ failure. Severe hypoxia is strongly associated with higher mortality rates, particularly in patients suffering from ARDS. Additionally, hypoxia can impair brain function, leading to symptoms such as confusion and agitation, which complicate the management of patients (38, 39).
Hypoxia, a frequent complication of COVID-19, is also closely linked to depressive and anxious states (28, 40). Elevated CK and LDH levels have emerged as early markers of COVID-19 infection, correlating with disease severity (41, 42). Recent studies suggest that even moderate hypoxia can trigger depression, anxiety, and cognitive impairments (40). It is possible that hypoxia induced by COVID-19 may directly lead to depression by disrupting neuronal energy homeostasis. Supporting this theory, hypoxia has been shown to increase plasma CK and LDH levels (43-45).
Moreover, the psychological stress associated with COVID-19 may further contribute to the development of depression by triggering glucocorticoid secretion (46). Glucocorticoids can disrupt neuronal energy homeostasis and glucose metabolism, both of which play key roles in the pathogenesis of depression (47-49).
Economic downturns often impact men more severely, as observed during the COVID-19 pandemic. Job loss, financial instability, and the pressure to fulfill traditional provider roles can heighten men's stress and anxiety. Studies indicate that job insecurity and unemployment are strongly linked to adverse psychological outcomes in men, contributing to increased depression rates (50, 51).
Men and women typically employ different coping strategies when dealing with stress. Men may be less likely to seek social support or express emotional distress, which can leave psychological issues unaddressed. In contrast, women often turn to social networks for emotional support, helping to alleviate stress. This difference may explain the higher prevalence of depression in men during COVID-19, as they may be less equipped to manage the psychological burden (51, 52).
Research suggests that men and women have distinct neurobiological responses to stress and trauma. The hypothalamic-pituitary-adrenal (HPA) axis may function differently based on gender, potentially increasing men's vulnerability to depression during stressful events like the pandemic. Furthermore, the inflammation and immune responses triggered by COVID-19 may exacerbate mental health issues, with men showing different inflammatory responses that influence mood disorders (51, 53).
5.1. Conclusions
In conclusion, our findings suggest that the mechanisms involved in metabolic support for neuronal physiological functions may be altered in COVID-19-infected patients, leading to depression. Glial fibrillary acidic protein, CK, and LDH levels appear to be associated with the severity of depression in COVID-19 patients. The simultaneous detection of elevated serum GFAP, CK, and LDH levels may serve as an indicator of depressive symptoms, particularly in males with COVID-19 infection.