1. Background
Acute lymphoblastic leukemia (ALL) is the most common cancer in children, accounting for 26% of pediatric blood malignancies, with the highest prevalence in the age group of 2 to 5 years (1). The recovery or cure rate for children with ALL is approximately 85%, but about 75% of these children experience treatment-related side effects, and 1 - 3% succumb to treatment toxicity (1). The management of childhood ALL involves multiple rounds of chemotherapy, beginning with induction therapy and advancing through consolidation and maintenance phases. The treatment duration typically spans around 2.5 years for females and 3.5 years for males (2).
Vincristine (VCR), a cornerstone chemotherapy medication in ALL treatment protocols, is utilized during the induction, consolidation, and maintenance phases (3). Vincristine binds to tubulin, disrupting microtubule polymerization and inducing apoptosis in cancer cells. However, it also impacts both small and large nerve fibers, leading to peripheral neuropathy (4). Specifically, Vincristine's binding to microtubule-forming tubulins in nerve fibers results in axon destruction, disruption of axon transmission, and subsequent peripheral neuropathy. This vincristine-induced peripheral neuropathy (VIPN) is the most prevalent and significant side effect of Vincristine administration (5).
Vincristine-Induced peripheral neuropathy presents with symptoms such as muscle weakness, areflexia, neuropathic pain, sensory loss, or autonomic polyneuropathies. These symptoms often necessitate dose reductions, treatment delays, or interruptions, significantly impacting the overall quality of life for children undergoing treatment (6). Nearly all children treated with VCR experience some degree of neuropathy (7, 8). While mild neuropathy allows treatment to continue until completion, 25 - 30% of cases present severe complications that interfere with daily activities, prompting dose reductions or, in some instances, cessation of treatment (9).
Vincristine-induced neurotoxicity manifests as paresthesia, weakened reflexes, muscle weakness, and autonomic neuropathy, affecting both sensory and motor neuron fibers (3). These complications typically emerge after several weeks of treatment, following the cumulative dose, but they can also occur after the initial dose (10). Although VIPN is generally reversible, symptom improvement is gradual and can take several months (11, 12). Symptomatic treatment often involves analgesics such as gabapentin and amitriptyline (13). However, the most effective approach to managing VIPN involves reducing the Vincristine dose, despite the potential disruption to the therapeutic process (6).
Previous studies have investigated the effectiveness of treatments for VINP using agents such as pyridoxine and pyridostigmine (14), glutamic acid (15), and glutamine (16). However, the potential efficacy of melatonin in addressing this condition has not yet been evaluated.
Melatonin, an indoleamine secreted by the pineal gland, plays a crucial role in regulating sleep-wake cycles, metabolic processes, and exhibiting preventive effects against oxidative stress (17-19). Melatonin has demonstrated effectiveness in improving neuropathy and has been studied as a neuroprotective agent in various conditions, including Alzheimer’s disease, hemorrhagic stroke, neonatal care for premature infants, and multiple sclerosis (MS) (20, 21). Numerous studies have highlighted melatonin’s ability to mitigate the side effects of chemotherapy drugs in both animal models and human cases (22, 23). Given its favorable safety profile for pediatric use, melatonin presents itself as a promising therapeutic candidate for children experiencing VINP.
2. Objectives
This study aims to evaluate the efficacy of melatonin in treating VINP in children diagnosed with ALL.
3. Methods
3.1. Study Design
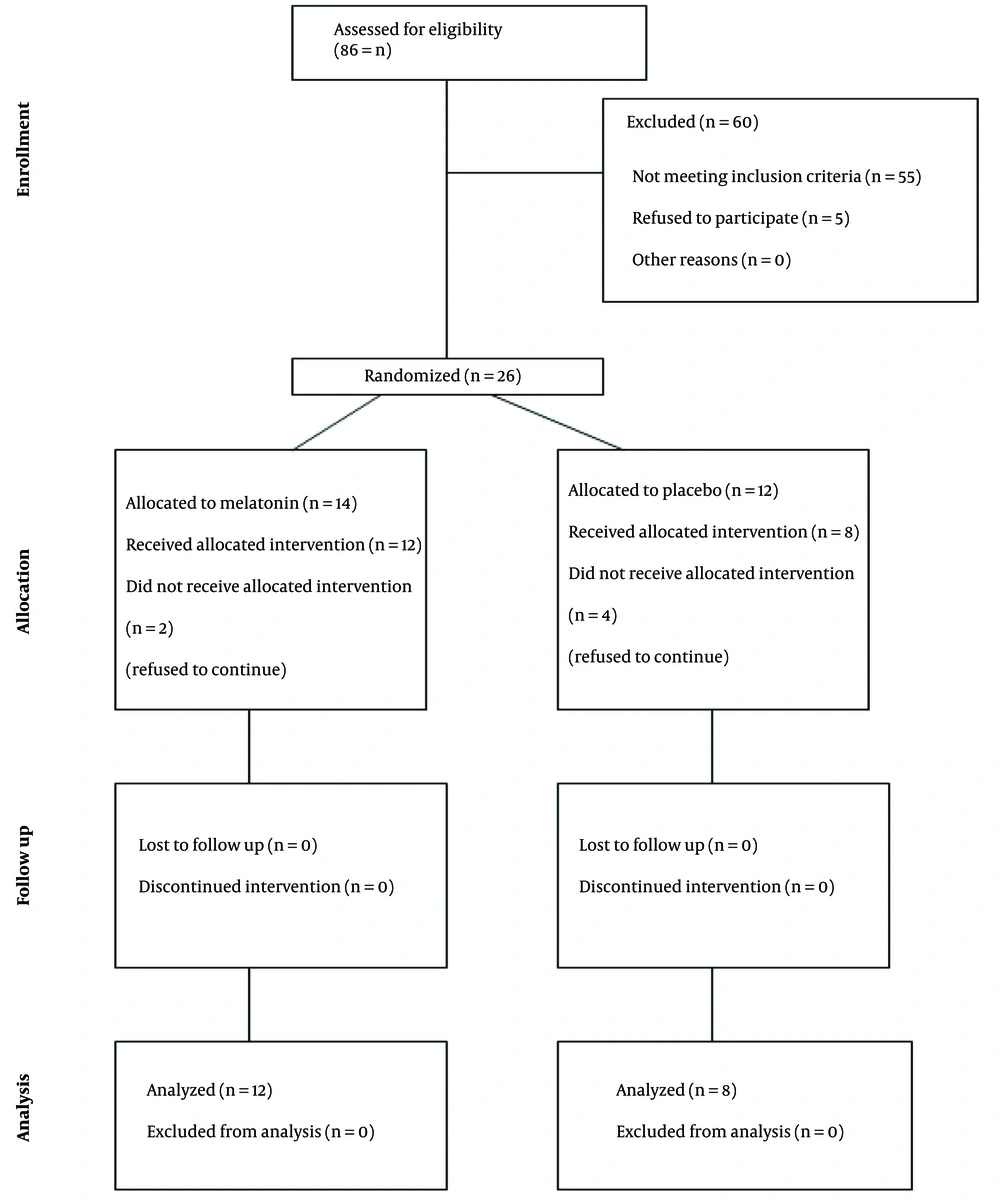
This study was a randomized, controlled, double-blind trial conducted in accordance with the Consolidated Standards of Reporting Trials (CONSORT) guidelines (Figure 1). The research was carried out in the hematology department of the Children's Medical Center Hospital, Tehran, Iran. Institutional Review Board (IRB) approval was obtained from Tehran University of Medical Sciences (IR.TUMS.TIPS.REC.1399.040), School of Medicine, along with approval from the Iranian Registry of Clinical Trials (IRCT20200710048068N1). Informed consent forms were signed by the eligible children's parents prior to their participation in the study.
3.2. Randomization and Blinding
Patients were randomly assigned to either the control or treatment groups using the permuted block method by a biostatistician. Each patient was assigned a code, and the corresponding code number, along with melatonin or placebo pills, was placed in opaque, sealed envelopes. These envelopes were opened in front of the patients. The principal investigators were not involved in the assignment process, and the biostatistician who analyzed and reported the results was blinded to the group assignments and the assignment process.
3.3. Procedures and Treatments
According to the center's protocol, the vincristine dosage in each of the four phases (induction, consolidation, maintenance, and intensification) is 1.5 mg/m², administered intravenously. During the 4-week induction phase, vincristine is injected weekly. In the 4-week consolidation phase, vincristine is administered on the first day. In the 8-week maintenance phases 1 and 2, vincristine is injected on days 1, 11, 21, 31, and 41. During the 8-week intensification phase, vincristine is injected on days 1, 8, and 15. In maintenance phase 3, which spans two years in quarterly cycles, vincristine is injected on days 1, 29, and 57. Melatonin was administered at a dose of 3 milligrams for individuals weighing less than 40 kg or 5 milligrams for those weighing more than 40 kg, once daily for one month. Patients in the placebo group received pills of identical appearance for one month. Patients in both groups also received dexamethasone as follows: In the induction phase (4 weeks), dexamethasone was administered at 6 mg/m²/day orally for 28 days; in the intensification phase (8 weeks), dexamethasone was given at 10 mg/m² on days 1 - 7 and 15 - 21; in the maintenance phase, which lasted for 2 years in 12-week cycles, dexamethasone was administered at 3 mg/m² on days 1 - 5, 29 - 33, and 57 - 61.
3.4. Participants, Inclusion and Exclusion Criteria
All children aged 5 to 12 years with ALL and clinically diagnosed VINP were included in the study. VINP was diagnosed using the pediatric-modified total neuropathy score (24). Exclusion criteria included hypersensitivity to melatonin, age over 12 years, central nervous system tumors, other neuromuscular disorders, disease recurrence (relapse), unstable hemodynamic conditions, or amputation of the upper or lower limbs.
3.5. Measurement of Outcomes
3.5.1. Pediatric-Modified Total Neuropathy Score
The pediatric-modified total neuropathy score (PED-MTNS) comprises three sets of inquiries related to sensory symptoms and pain, motor function, and autonomic function, along with a five-part neurological examination. This examination includes assessments of light touch, vibration sensation, pin sensation, distal strength through manual muscle testing, and deep tendon reflexes. This comprehensive evaluation covers various aspects of the peripheral nervous system (PNS). Each item in both the questionnaire and physical examination is scored from 0 (indicating no symptoms) to 4 (indicating severe symptoms). The most severe scores from the three questionnaire items, combined with the scores from the five physical examination items, are summed to calculate the total PED-MTNS score, which ranges from 0 to 32. Children with a total PED-MTNS score of 5 or above were classified as having VIPN (24).
3.5.2. CTCAE Score
The CTCAE criteria used to evaluate VIPN include assessments of constipation, peripheral sensory neuropathy, peripheral motor neuropathy, and neuralgia. Scores range from 0 (indicating no symptoms) to 3 (indicating severe symptoms), with category 4 designated as life-threatening and category 5 representing a fatal outcome in cases of lethal adverse events. The maximum cumulative CTCAE score achievable across these four items is 18. Individuals with a total CTCAE score of 2 or above are classified as having VIPN (4).
3.5.3. PedsQL Child and Parent Score
The PedsQL is a validated assessment tool designed to measure health-related quality of life in pediatric patients, incorporating both self-report and parent proxy-report measures. A 5-point response scale is used for the child self-report (ages 8 to 18) and parent proxy-report, with responses ranging from 0 (indicating "never a problem") to 4 (indicating "almost always a problem"). For younger children (ages 5 to 7), a simplified 3-point response scale is applied, ranging from 0 ("not at all a problem") to 4 ("a lot of a problem").
Items are reverse-scored and linearly transformed to a 0 - 100 scale (where 0 = 100, 1 = 75, 2 = 50, 3 = 25, and 4 = 0), ensuring that higher scores reflect better health-related quality of life. The multidimensional PedsQL 3.0 Cancer Module Acute Version includes 27 items categorized into eight scales: (1) pain and hurt (2 items), (2) nausea (5 items), (3) procedural anxiety (3 items), (4) treatment anxiety (3 items), (5) worry (3 items), (6) cognitive problems (5 items), 7) perceived physical appearance (3 items), and (8) communication (3 items) (25).
3.6. Statistical Analysis
SPSS version 26 was used for all statistical analyses. The mean scores of all continuous variables were compared between the two groups using the Mann-Whitney U test, while repeated measures ANOVA was employed to analyze mean changes over time between groups (eta squared was calculated as the effect size). All demographic variables and VCN doses were compared between groups, and if any significant differences were identified, the variable was considered a covariate and adjusted in all analyses. A P-value of < 0.05 was considered statistically significant.
4. Results
A total of 20 patients were included in the study (mean age = 7.45 ± 2.31 years; 55% female), with 60% allocated to the treatment group. No significant differences were observed in baseline clinical or demographic data (Table 1). Of the 20 patients, 15% were in the induction phase, 10% in the consolidation phase, 55% in the maintenance phase, and 20% in the intensification phase. Specifically, in the treatment group, 15% were in the induction phase, 30% in the maintenance phase, and 15% in the intensification phase. Meanwhile, in the placebo group, 10% were in the consolidation phase, 25% in the maintenance phase, and 5% in the intensification phase.
| Variables | All (N = 20) | Placebo (N = 8) | Melatonin (N = 12) | P-Value |
|---|---|---|---|---|
| Gender | 0.670 | |||
| Female | 11 | 5 (45.5) | 6 (54.5) | |
| Male | 9 | 3 (33.3) | 6 (66.7) | |
| Admission | 0.167 | |||
| Outpatient | 8 | 5 (62.5) | 3 (37.5) | |
| Inpatient | 12 | 3 (25.0) | 9 (75.0) | |
| VCR Route | N/a | |||
| Bolus | 20 | 8 (40.0) | 12 (60.0) | |
| Continuous Infusion | 0 | 0 (0.0) | 0 (0.0) | |
| Chemo Phase | 0.181 | |||
| Induction | 3 | 0 (0.0) | 3 (100.0) | |
| Consolidation | 2 | 2 (100.0) | 0 (0.0) | |
| Maintenance | 11 | 5 (45.5) | 6 (54.5) | |
| Intensification | 4 | 1 (25.0) | 3 (75.0) | |
| Age | 7.45 ± 2.31 | 7.25 ± 1.91 | 7.58 ± 2.61 | 0.875 |
| Weight | 21.40 ± 6.29 | 22.75 ± 7.23 | 20.50 ± 5.74 | 0.438 |
| VCR dose | 1.18 ± 0.38 | 1.31 ± 0.29 | 1.09 ± 0.41 | 0.226 |
| PED-MTNS score 1 | 9.70 ± 2.72 | 9.38 ± 2.62 | 9.92 ± 2.87 | 0.693 |
| PED-MTNS score 2 | 6.20 ± 3.40 | 6.88 ± 2.36 | 5.75 ± 3.98 | 0.348 |
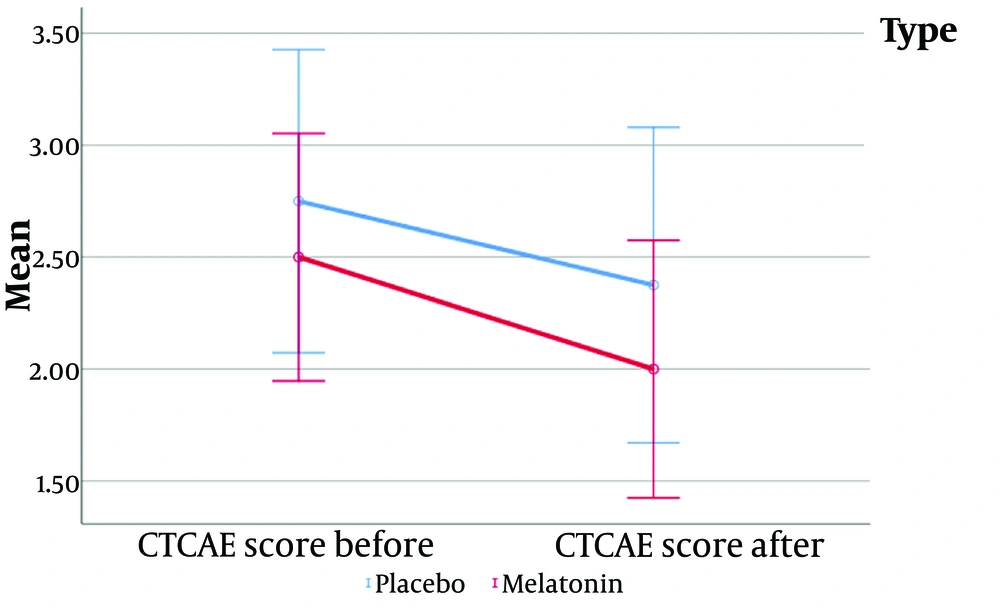
| CTCAE score 1 | 2.60 ± 0.94 | 2.75 ± 1.04 | 2.50 ± 0.90 | 0.516 |
| CTCAE score 2 | 2.15 ± 0.99 | 2.38 ± 0.74 | 2.00 ± 1.13 | 0.162 |
| PedsQL parent score 1 | 57.52 ± 12.04 | 62.28 ± 15.50 | 54.36 ± 8.37 | 0.165 |
| PedsQL parent score_2 | 61.66 ± 10.80 | 62.53 ± 13.51 | 61.09 ± 9.19 | 0.877 |
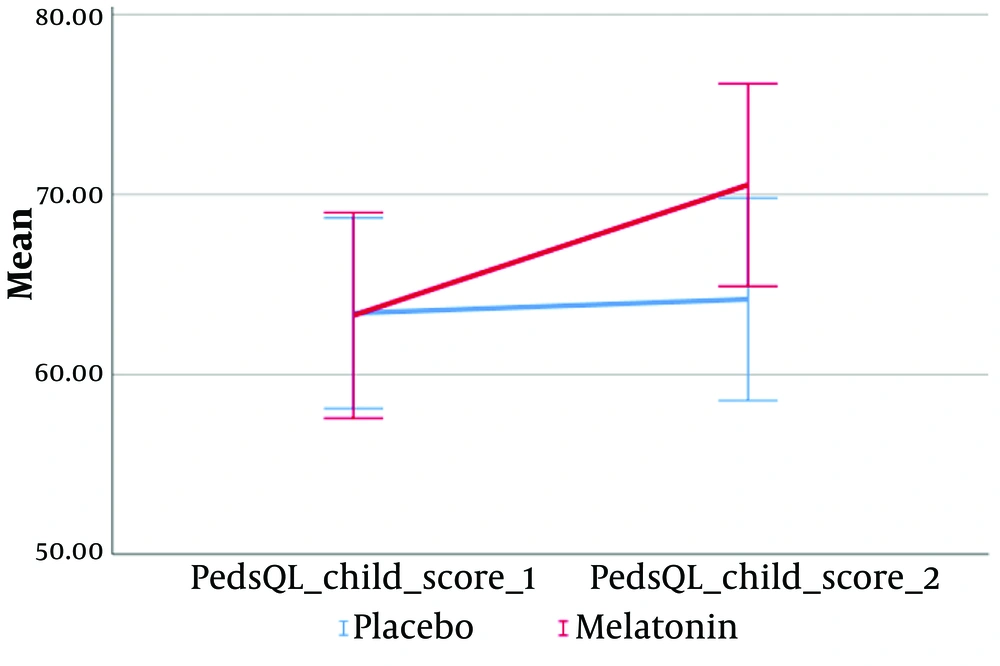
| PedsQL child score_1 | 63.33 ± 8.80 | 63.40 ± 7.49 | 63.28 ± 9.90 | 0.938 |
| PedsQL child score 2 | 67.99 ± 9.42 | 64.18 ± 7.96 | 70.53 ± 9.76 | 0.189 |
| Pain child 1 | 4.55 ± 1.88 | 4.88 ± 1.73 | 4.33 ± 2.02 | 0.636 |
| Pain child 2 | 2.80 ± 2.46 | 4.00 ± 2.39 | 2.00 ± 2.26 | 0.041 |
| VCR cumulative dose | 21.92 ± 15.78 | 26.46 ± 18.31 | 18.90 ± 13.85 | 0.354 |
Abbreviations: VCR, vincristine; PED-MTNS, pediatric-modified total neuropathy score.
a Values are expressed as mean ± SD or No. (%).
The CTCAE score was used to measure the main outcome, and while both groups demonstrated a reduction in CTCAE scores, no significant difference was observed between them.
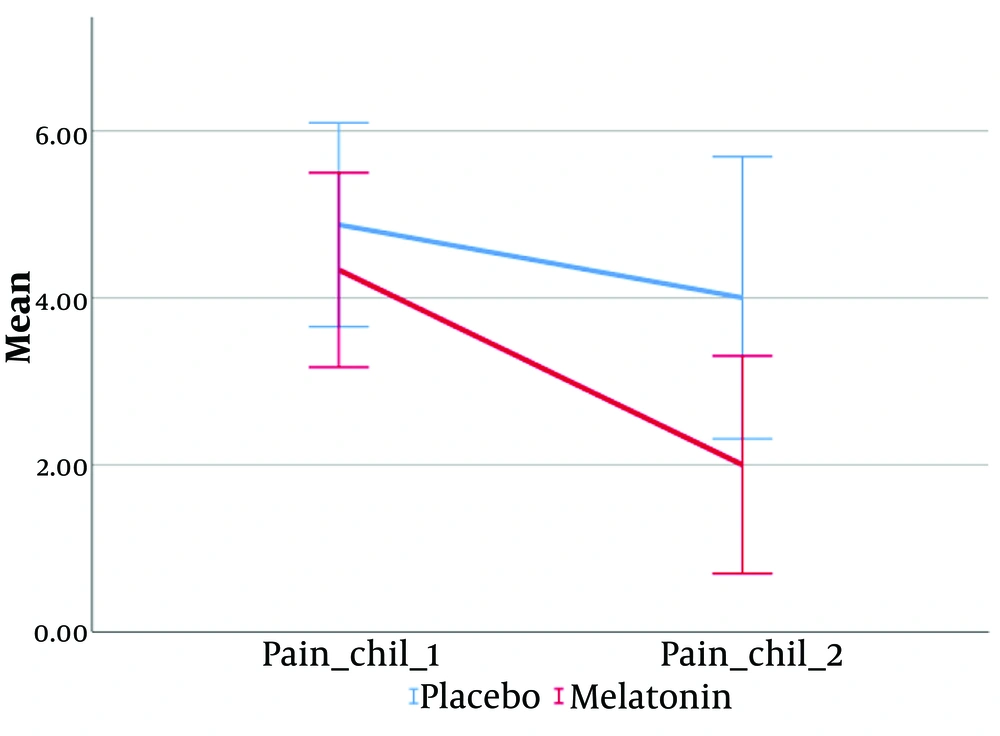
The only significant difference observed was in the pain score after the intervention, with children in the placebo group having a significantly higher mean pain score compared to the treatment group (4 ± 2.39 vs. 2 ± 2.26, P = 0.041).
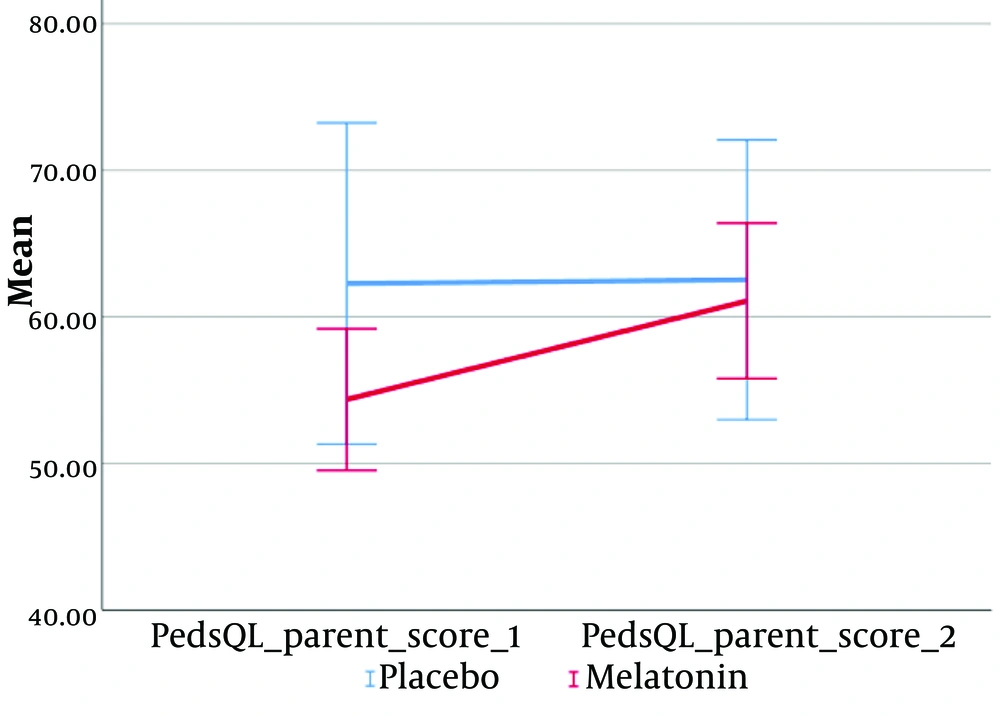
According to the results of repeated measures ANOVA (Figures 2-5), a significant interaction effect was identified between the intervention group and the following variables: PedQL child scores (Greenhouse-Geisser = 100.67, F (1, 18) = 24.99, P < 0.001), PedQL Parent scores (Greenhouse-Geisser = 100.67, F (1, 18) = 26.93, P < 0.001), and pain scores (Greenhouse-Geisser = 5.10, F (1, 18) = 10.47, P = 0.005). This indicates that the intervention had a substantial and statistically significant impact on the quality of life of the children, their parents, and the children's pain scores.
The effect size (eta squared) suggests that 58.1% of the variance in PedQL child scores, 59.9% in PedQL parent scores, and 36.8% in pain scores over time can be attributed to the intervention group.
5. Discussion
Vincristine is a crucial component of many pediatric oncology treatment protocols and has been used in children for several decades. However, VIPN is a serious side effect that affects cancer patients undergoing chemotherapy regimens containing this drug. Vincristine-induced peripheral neuropathy can persist long after the cessation of treatment, sometimes necessitating dose reductions or even the discontinuation of therapy. Symptoms of VIPN typically manifest between 2 and 19 weeks after the initiation of vincristine therapy (26).
Most patients display early signs of toxicity when they receive cumulative doses of 5 - 6 mg/m² of VCR, although significant toxicity is generally not observed at cumulative doses below 15 - 20 mg/m² (27). In our study, the average total cumulative dose of VCR was 21.92 ± 15.78 mg/m². Vincristine-induced peripheral neuropathy is generally reversible, but the recovery process is gradual and may take several months. Furthermore, symptoms can continue to progress for several months before showing signs of improvement.
In a randomized, single-blinded, placebo-controlled clinical trial, Mokhtar et al. (28) evaluated the role of glutamic acid in preventing VIPN among pediatric patients receiving vincristine during the 4-week induction course. They conducted physical and neurological examinations to record, monitor, and grade neurotoxic symptoms. Their findings indicated a reduction in neurotoxicity in the treatment group. The ages of the participants ranged from 3 to 18 years.
Similarly, Bradfield et al. (15) conducted a randomized, double-blind, placebo-controlled clinical trial to assess the potential of glutamic acid in preventing VIPN among 250 children diagnosed with Wilms' tumor, rhabdomyosarcoma, non-Hodgkin's lymphoma, and ALL. Glutamic acid was administered before the first dose of vincristine. The study found that glutamic acid was effective in preventing VIPN only in patients aged 13 years or older, with no significant benefits observed in preadolescent patients.
These studies have explored the role of glutamic acid as a preventive measure against the occurrence of VIPN. However, the present study focuses solely on evaluating the effectiveness of melatonin after the onset of neuropathy. Given melatonin's preventive effects against oxidative stress and its neuroprotective properties, future research could investigate its potential role in preventing this complication. Considering the influence of age on the occurrence of VIPN, as highlighted in this study compared to the previous ones, the age range of patients was limited. Additionally, the PED-MTNS score, recognized for its higher validity, was employed in diagnosing neuropathy, ensuring a more accurate assessment within this study.
According to a study by Van de Velde et al. (29), vincristine-induced neuropathy significantly diminishes the quality of life in children undergoing treatment for malignancies, underscoring the critical impact of neuropathy in this population. The exact mechanism and pathology of this condition remain unclear, and no effective pharmacological intervention has been identified to prevent or treat this side effect. Existing reports of successful pharmacological measures are limited to a few case studies, such as the use of pyridoxine and pyridostigmine for cranial neuropathy. Despite vincristine’s pivotal role in cancer treatment regimens, measures to mitigate its neuropathic effects are essential (30). Given the need to optimize vincristine-based treatments while reducing VIPN and maintaining therapeutic efficacy, this study aimed to evaluate the effectiveness of melatonin in treating VINP in children with ALL.
In a study conducted by Lavoie Smith et al. (31), it was observed that severe neuropathy peaked in the fourth month of treatment, two months after reaching the maximum dose density of vincristine. During months 2, 6, and 10, the PED-MTNS scores peaked, reflecting increased neuropathy levels. In the present study, 55% of the patients were in the maintenance phase and displayed neuropathic symptoms. This phase involved higher cumulative vincristine doses compared to the induction and consolidation phases, correlating with a greater incidence of neuropathy. Previous research (4, 9, 31-37) has similarly established a relationship between the frequency of neuropathy and the cumulative vincristine dosage, with higher doses during the maintenance phase leading to an increased likelihood of neuropathic symptoms.
To include patients with a potential for neuropathy, all participants underwent thorough clinical examinations to ensure accuracy and prevent deviations in the study. Neuropathy symptoms are progressive, beginning in the hands and feet and advancing toward the arms and legs. Foot drop is typically the first manifestation of peripheral neuropathy. Examinations included assessments of Achilles and patellar tendon reflexes, weakness in lower limb muscles, and autonomic symptoms such as jaw pain, abdominal pain, constipation, and urinary retention. The primary purpose of these comprehensive clinical evaluations was to confirm that neuropathy was attributable to vincristine.
This distinction is particularly critical because, during the induction phase, high doses of dexamethasone are administered alongside vincristine as part of the chemotherapy regimen. Dexamethasone is known to have myopathy as a common side effect, which could potentially complicate the identification of neuropathy. According to a study by de Vries et al. (38), such side effects significantly impact the quality of life in children undergoing cancer treatment.
One of the strengths of this study is the comprehensive measurement of VIPN, achieved through the use of two assessment tools, including physical examinations conducted at two different points during treatment. These evaluations were performed by a neurologist, ensuring accuracy and reliability. Currently, the pediatric-modified total neuropathy score (PED-MTNS) is the recommended tool for assessing VIPN in children. Previous studies have demonstrated that PED-MTNS is more sensitive for diagnosing VIPN compared to the CTCAE evaluation.
This greater sensitivity might explain why the effect of melatonin on children with VIPN, as measured by PED-MTNS, was not significant compared to CTCAE in the treatment group. Additionally, the stronger association between VIPN and its negative impact on health-related quality of life (HR-QoL) when assessed using ped-MTNS may also contribute to these findings. The adverse effects of VIPN on HR-QoL appear to be more pronounced when the severity of VIPN is higher.
This study demonstrates that, despite employing two evaluation methods—CTCAE and PED-MTNS—for assessing VIPN, the administration of melatonin at a dose of 3 mg for one month did not significantly improve VIPN in children with ALL. However, melatonin was effective in enhancing the quality of life for these children and contributed to pain reduction during the one-month treatment period. Pain experienced during cancer treatment greatly impacts quality of life.
Notably, in the quality-of-life questionnaire, the items related to pain and discomfort that were most frequently reported may not have been exclusively associated with VIPN; other causes of pain could also be factors. Additionally, findings from the children's oncology group trial suggest that symptoms of myopathy caused by corticosteroids might have obscured the potential benefits of melatonin for vincristine-induced neuropathy.
The prevalence of ALL in children is highest in the age range of 2.5 to 5 years, an age group where children are often unable to articulate symptoms of neuropathy, underscoring the critical importance of clinical examinations by physicians. The PED-MTNS questionnaire is validated for use in children older than 5 years, which limited the study population and extended the time required for sample collection. Consequently, there is a need for an easy and practical method to identify neuropathy in children under 5 years of age within clinical settings.
During the study period, sample collection was further constrained by several factors, including the target age range of 5 to 12 years for children with ALL experiencing moderate to severe neuropathy, the reluctance of some parents to participate, and a decline in visits to the children's medical center due to the COVID-19 pandemic. These limitations in sample size could potentially affect the results of the data analysis for the PED-MTNS and CTCAE questionnaires.
5.1. Limitations and Suggestion for Future Research
Considering that VIPN measurements were assessed by physicians while the quality of life was evaluated based on responses from the patient or their parents, this disparity could lead to differing interpretations of VIPN by the physician and the patient. Vincristine-induced peripheral neuropathy was evaluated using physical examinations and questionnaires, whereas the quality of life was assessed solely through a questionnaire completed by the patient or their parents, not the physician. Additionally, the quality of life is influenced by the coping mechanisms and adaptability of the child and their family. Factors such as poverty and social isolation exacerbate and perpetuate distress.
Overall, it has been shown that health inequalities significantly impact both the health and adaptability of individuals in pediatric oncology. Family structure, beliefs, and coping abilities play natural roles in managing and adapting to cancer-related challenges. Thus, screening for these psychosocial factors in families is crucial. Oncology care should take these psychosocial dimensions into account, focusing not only on physical health but also on social and psychological aspects of the patient's and family's experience.
When using the same questionnaire for clinical examination, different diagnoses may be reached for similar patients. However, in our study, all patients were clinically evaluated by a neurologist both before and after the intervention, ensuring that the relevant questionnaires were assessed solely by a specialist to ensure reliability. Both questionnaires were completed by each patient on the same day. To minimize errors during the completion of questionnaires for neuropathy and quality of life assessments, questions were asked uniformly for each patient.
Given the significant time and accuracy required for each questionnaire to be completed by the researcher at the clinic or bedside, which can sometimes be challenging, the development and implementation of simpler measurement methods to assess the neuropathy status of children in healthcare settings are necessary. Factors that limit the widespread use of PED-MTNS include the need for trained personnel to administer assessments, the time and clinical space required for evaluations, occasional discomfort associated with certain examination methods (e.g., pinpricks), and the limited ability of some children to focus and cooperate during the lengthy PED-MTNS-based examination.
5.2. Conclusions
In conclusion, this study highlights the critical need to address VIPN in children undergoing vincristine treatment for cancer and to enhance their quality of life. Given melatonin's mechanism of action, exploring its potential role in preventing the occurrence of VIPN in children undergoing treatment could prove beneficial. Further research in this area is necessary. Future studies should consider using higher doses of melatonin, larger sample sizes, and longer study durations to provide more comprehensive insights. Additionally, all children receiving vincristine treatment should be routinely evaluated for the severity of neuropathy to ensure timely intervention and management.