1. Background
Stroke, also known as a cerebrovascular accident (CVA), is a medical condition characterized by the sudden death of specific brain cells due to a complete blockage of oxygen supply. This condition typically results from damage, rupture, or obstruction of blood vessels in the brain (1, 2). Globally, stroke is a major concern for medical professionals and healthcare providers, ranking as the second leading cause of mortality and the third leading cause of disability (3). Approximately 70% of all strokes, along with 87% of stroke-related deaths and disability-adjusted life years, occur in low- and middle-income countries (4-6). In 2019, Iran reported 963,512 cases of stroke and 102,778 associated fatalities (7).
During acute ischemic stroke (AIS), the integrity of the blood-brain barrier (BBB) is disrupted, undergoing structural changes that exacerbate brain injury. Extensive research has identified oxidative stress, protease activation, and infiltration of circulating leukocytes as key mechanisms that damage the BBB and contribute to hemorrhagic transformation (HT), particularly following recanalization induced by tissue-type plasminogen activator (t-PA) (8). Currently, recanalization with t-PA remains the only pharmacological treatment for ischemic stroke (9, 10). Intravenous r-tPA must be administered within 4.5 hours of stroke symptom onset, while mechanical thrombectomy is recommended within 6 hours (11). To minimize the risk of HT—a serious complication of intravenous thrombolysis—t-PA is administered under strict clinical guidelines (12).
The high global incidence of CVA and its associated complications remains a significant challenge for the medical community. Hemorrhagic transformation, a critical and potentially life-threatening complication, is particularly concerning in patients treated with r-tPA for stroke.
2. Objectives
This study aims to identify factors influencing the extent of cerebral hemorrhage in stroke patients receiving r-tPA.
3. Methods
3.1. Study Design and Patient Selection
This descriptive-analytical cross-sectional study was conducted at Shahid Beheshti Hospital, Qom, Iran, from April 2019 to March 2020, following approval from the Ethics Committee of QUMS (IR.MUQ.REC.1401.188). The minimum sample size was calculated based on the study by Dharmasaroja et al. (13), assuming a bleeding prevalence of 13% and a 5% margin of error. A total of 175 cases were included. Patients were selected through census sampling from all stroke patients presenting to the emergency department and receiving r-tPA, provided they met the inclusion and exclusion criteria.
Inclusion criteria included patients with a confirmed diagnosis of AIS, no contraindications for thrombolytic therapy, and symptom onset within 4.5 hours prior to treatment. Patients with incomplete data, hemorrhagic stroke, alternative final diagnoses (e.g., stroke mimics), or death prior to intervention were excluded.
Written informed consent was obtained from all participants or their legal representatives prior to inclusion in the study. For patients who were unable to provide consent due to their medical condition, consent was obtained from their next of kin or legal guardian. Confidentiality and anonymity of patient data were strictly maintained throughout the research process.
3.2. Data Collection
Patient data were extracted from medical records and documented in pre-prepared questionnaires in compliance with ethical guidelines and confidentiality standards. Data collected included demographic information (age, sex), history of underlying diseases (diabetes, hypertension, dyslipidemia), medication use (antiplatelets, anticoagulants), and laboratory results. Laboratory parameters included coagulation factors such as prothrombin time (PT), partial thromboplastin time (PTT), International Normalized Ratio (INR), fibrinogen, and complete blood count (CBC) components, including white blood cell count, neutrophil and lymphocyte percentages, hemoglobin levels, and platelet count.
When information gaps were identified, families of the patients were contacted to obtain the required data. Bleeding complications, particularly intracerebral hemorrhage (ICH) following thrombolysis, were closely monitored. All patients underwent clinical examinations and follow-up CT scans within 24 hours of r-tPA administration. In addition, blood samples were collected six hours post-thrombolysis to assess coagulation status and potential early markers of HT.
3.3. Statistical Analysis
Statistical analyses were conducted using SPSS software (version 22). Data were presented as mean ± standard deviation (SD) for normally distributed variables and as median with interquartile range (IQR) for non-normally distributed variables. Comparative analyses were performed using receiver operating characteristic (ROC) curve analysis, chi-square test, t-test, and logistic regression. Statistical significance was set at P < 0.05.
4. Results
This study investigated the incidence and determinants of HT within 24 hours in patients diagnosed with AIS who received r-tPA. Out of 175 patients included in the study, 28 (16.0%) experienced HT following treatment. Among the total population, 61 (34.9%) were female, and 114 (65.1%) were male. The mean and standard deviation of age were analyzed for patients in both groups. The results showed that the mean age of patients with evidence of HT was comparable to that of patients without HT following intravenous r-tPA (66.96 ± 13.74 years vs. 64.53 ± 13.70 years, respectively) (Table 1).
| Characteristics | Without ICH | With ICH | P-Value |
|---|---|---|---|
| Total patients | 147 (84) | 28 (16) | |
| Age, median (Q1; Q3) | 64 (58; 74) | 66 (60; 77) | 0.391 |
| Sex | 0.742 | ||
| Male | 95 (54.2) | 19 (10.8) | |
| Female | 52 (29.7) | 9 (5.3) | |
| Co-morbid diseases | |||
| Diabetes melitus | 0.040 | ||
| Yes | 35 (20) | 12 (6.9) | |
| No | 112 (64) | 16 (9.1) | |
| Hypertension | 0.005 | ||
| Yes | 86 (49.2) | 25 (14.3) | |
| No | 61 (34.8) | 3 (1.7) | |
| Hyperlipidemia | 0.0006 | ||
| Yes | 17 (9.7) | 11 (6.2) | |
| No | 130 (74.4) | 17 (9.7) | |
| Past Cerebrovascular accidents | 0.012 | ||
| Yes | 26 (14.9) | 11 (6.2) | |
| No | 121 (69.2) | 17 (9.7) | |
| Cardiovascular diseases | 0.001 | ||
| Yes | 36 (20.6) | 15 (8.6) | |
| No | 111 (63.4) | 13 (7.4) | |
| Antiplatelet/anticoagulant drug use | |||
| Antiplatelet | 0.006 | ||
| Yes | 41 (23.5) | 16 (9.1) | |
| No | 106 (60.5) | 12 (6.9) | |
| Anticoagulant | 0.009 | ||
| Yes | 7 (4) | 6 (3.5) | |
| No | 140 (80) | 22 (12.5) |
Abbreviations: ICH, intracerebral hemorrhage.
a Values are expressed as No. (%) unless otherwise indicated.
The medical history of diabetes mellitus, hypertension, dyslipidemia, cardiovascular diseases, and prior CVA was analyzed. The results indicated a statistically significant relationship between these variables and HT (P < 0.05) (Table 2). In addition to patients' medical history, the use of antiplatelet and anticoagulant drugs was also examined in relation to HT. The results showed that most patients had no history of anticoagulant or antiplatelet drug use. However, a statistically significant relationship was observed between antiplatelet drug use and HT following intravenous r-tPA (P = 0.005) (Table 3).
| Underlying Disease | Odd’s Ratio | Confidence Interval (95%) | P-Value |
|---|---|---|---|
| Diabetes mellitus | 2.40 | 1.04 - 5.55 | 0.040 |
| Hypertension | 5.91 | 1.707 - 20.460 | 0.005 |
| Hyperlipidemia | 4.94 | 1.988 - 12.310 | 0.0006 |
| Past cerebrovascular accidents | 3.01 | 1.263 - 7.178 | 0.012 |
| Cardiovascular diseases | 3.85 | 1.652 - 8.991 | 0.001 |
| Anti-platelets | 3.231 | 1.394 - 7.488 | 0.006 |
| Anti-coagulant | 5.340 | 1.501 - 18.999 | 0.009 |
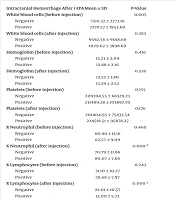
| Intracranial Hemorrhage After r-tPA | Mean ± SD | P-Value |
|---|---|---|
| White blood cells (before injection) | 0.605 | |
| Negative | 7516.32 ± 2273.16 | |
| Positive | 7278.57 ± 1953.60 | |
| White blood cells (after injection) | 0.383 | |
| Negative | 9592.56 ± 9018.68 | |
| Positive | 11129.62 ± 3898.69 | |
| Hemoglobin (before injection) | 0.416 | |
| Negative | 13.23 ± 2.04 | |
| Positive | 12.88 ± 2.16 | |
| Hemoglobin (after injection) | 0.578 | |
| Negative | 13.52 ± 1.80 | |
| Positive | 13.29 ± 2.52 | |
| Platelets (before injection) | 0.155 | |
| Negative | 209394.55 ± 68329.23 | |
| Positive | 231489.28 ± 103897.70 | |
| Platelets (after injection) | 0.176 | |
| Negative | 201404.95 ± 75833.54 | |
| Positive | 224518.51 ± 95878.27 | |
| % Neutrophil (before injection) | 0.460 | |
| Negative | 60.90 ± 11.16 | |
| Positive | 62.57 ± 9.09 | |
| % Neutrophil (after injection) | 0.000 a | |
| Negative | 70.79 ± 11.86 | |
| Positive | 80.07 ± 7.66 | |
| % Lymphocytes (before injection) | 0.243 | |
| Negative | 31.10 ± 10.27 | |
| Positive | 28.68 ± 7.97 | |
| % Lymphocytes (after injection) | 0.000 a | |
| Negative | 22.01 ± 10.57 | |
| Positive | 13.00 ± 5.72 | |
| PT (before injection) | 0.048 a | |
| Negative | 13.20 ± 1.06 | |
| Positive | 12.77 ± 0.81 | |
| PT (after injection) | 0.051 | |
| Negative | 13.97 ± 1.76 | |
| Positive | 12.86 ± 3.04 | |
| PTT (before injection) | 0.482 | |
| Negative | 30.51 ± 9.58 | |
| Positive | 29.03 ± 12.20 | |
| PTT (after injection) | 0.019 a | |
| Negative | 31.87 ± 7.33 | |
| Positive | 27.41 ± 7.76 | |
| INR (before injection) | 0.346 | |
| Negative | 1.12 ± 0.11 | |
| Positive | 1.09 ± 0.15 | |
| INR (after injection) | 0.033 a | |
| Negative | 1.18 ± 0.17 | |
| Positive | 1.07 ± 0.25 | |
| NLR (before injection) | 0.081 a | |
| Negative | 1.95 ± 0.21 | |
| Positive | 2.18 ± 0.29 | |
| NLR (after injection) | < 0.001 a | |
| Negative | 3.21 ± 0.36 | |
| Positive | 6.15 ± 0.68 |
Abbreviations: r-tPA, recombinant tissue plasminogen activator; NLR, neutrophil-to-lymphocyte ratio; INR, International Normalized Ratio; PTT, partial thromboplastin time.
a P < 0.05.
The findings revealed a significant increase in the average percentage of neutrophils following intravenous r-tPA compared to pre-injection levels (80.07% vs. 77.79%). Furthermore, the mean neutrophil proportion was higher in patients who developed HT after injection compared to those who did not (80% vs. 70%, respectively) (Table 3).
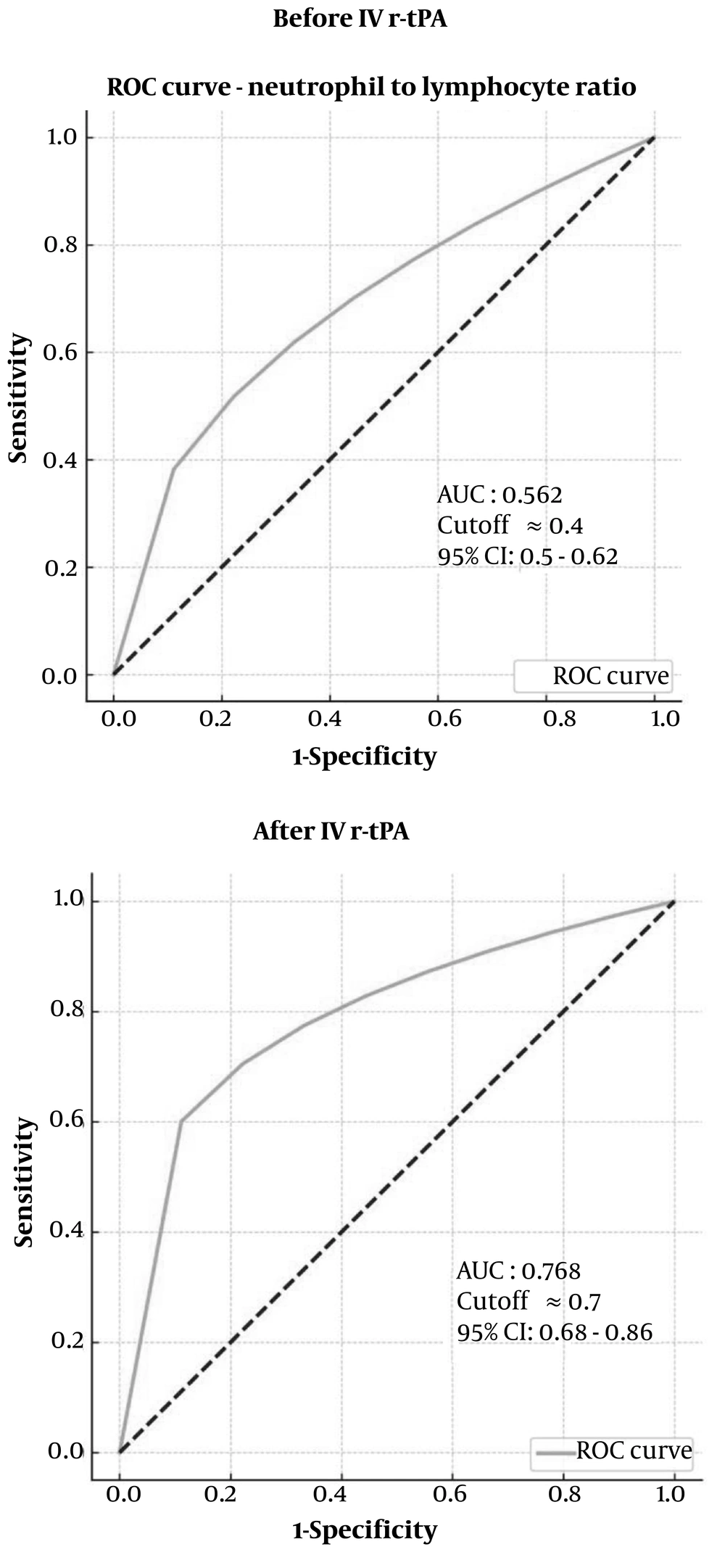
4.1. Neutrophil to Lymphocyte Ratio Receiver Operating Characteristic Curve Analysis
Neutrophil to lymphocyte Ratio ROC Curve Analysis has been shown in Figure 1.
4.1.1. Receiver Operating Characteristic Curve Analysis Before Recombinant Tissue Plasminogen Activator
- An AUC of 0.562 indicates that the model has poor discrimination. This means that the ability of the neutrophil-to-lymphocyte ratio (NLR) before r-tPA to distinguish between the two classes (e.g., outcome or condition) is only slightly better than random (AUC = 0.5).
- The confidence interval (0.5 - 0.62) shows that the performance may vary, but it is still within the range that suggests poor predictive power.
- A cutoff value of 0.4 indicates the point where the sensitivity and specificity are balanced; however, given the low AUC, the predictive reliability at this threshold is limited.
4.1.2. Receiver Operating Characteristic Curve Analysis After Recombinant Tissue Plasminogen Activator
- An AUC of 0.768 indicates a significant improvement in the model's performance after r-tPA administration. This value suggests fair to good discrimination, meaning that the NLR measured after r-tPA is much more effective at distinguishing between the two classes than the pre-treatment NLR.
- The confidence interval (0.68 - 0.86) demonstrates greater consistency and reliability in the model's performance, as it remains entirely above 0.5 and extends to 0.86.
- The cutoff value of 0.7 represents the threshold for optimizing sensitivity and specificity after r-tPA injection. With a higher AUC, this cutoff is more meaningful and likely reflects an improved balance between true positive and false positive rates.
4.1.3. Comparison
- Improvement in AUC: The NLR’s ability to predict the outcome significantly improves from an AUC of 0.562 (before r-tPA) to 0.768 (after r-tPA). This suggests that the NLR becomes a more valuable predictive biomarker following the intervention.
- Confidence Interval: The confidence interval after r-tPA is narrower and consistently above 0.5, indicating improved and more reliable predictive power.
- Practical Implication: The increase in AUC and a better-defined cutoff point after r-tPA indicate that monitoring NLR post-intervention provides more reliable diagnostic or prognostic information compared to pre-intervention levels.
The NLR demonstrates potential as a predictive biomarker for HT in patients with acute stroke. Although its predictive value may be limited prior to thrombolytic therapy, monitoring NLR following r-tPA administration offers improved insights into the likelihood of hemorrhagic complications. This finding suggests that post-treatment NLR assessments could enhance clinicians' ability to evaluate and manage HT risk, thereby facilitating more informed decision-making in the acute care of stroke patients.
5. Discussion
Hemorrhagic transformation is widely recognized as the most significant complication associated with intravenous thrombolysis in cases of AIS. Thrombolytic therapy with intravenous r-tPA is an effective treatment for AIS; however, studies have shown that this therapy may increase the risk of HT (14, 15). Given the potential risk of HT associated with thrombolysis using r-tPA, studies suggest that the likelihood of this complication may increase by 4 to 27 times (16).
It is important to note that ICH associated with r-tPA is categorized into two types: (1) Symptomatic (sICH), and (2) asymptomatic (aICH). According to previous research, the incidence of sICH following treatment with a standard dose of r-tPA ranges from 2% to 7% (17). One of the primary concerns for physicians when prescribing this medication is the risk of HT following therapy. Unfortunately, comprehensive information regarding the advantages and disadvantages of thrombolysis therapy in patients at the highest risk of HT remains unavailable (18). For this reason, the present study aimed to investigate the prevalence and contributing factors to the development of HT in patients undergoing thrombolysis therapy. The findings indicated that approximately 16% of the total population developed HT following intravenous r-tPA administration.
The Third International Stroke Trial (IST-3) (19) has documented an overall positive outcome of r-tPA treatment in elderly patients. Notably, the IST-3 trial included a significant proportion (53%) of individuals aged 80 and above, among whom no increased incidence of post-r-tPA HT was observed compared to those under 80 years of age. Furthermore, numerous studies have consistently demonstrated no significant difference in the occurrence of sICH between younger and older patients (20-23). Our study supports these findings by demonstrating that age is not a significant prognostic factor for HT. This highlights that individuals across various age groups can benefit therapeutically from treatment interventions. Hypertension, which is prevalent among patients with AIS, is closely associated with an increased risk of HT (24-26). Studies have shown that over 60% of individuals with AIS present with elevated blood pressure levels (27). Chronic hypertension significantly impacts the cerebral vasculature, leading to various alterations, including increased vascular resistance, enhanced BBB permeability, impaired endothelial function, and reduced efficiency of collateral circulation (28). In our study, a medical history of diabetes, hypertension, dyslipidemia, IHD, and prior CVA were identified as potential risk factors for the development of cerebral hemorrhage. Notably, the prevalence of these factors was higher in patients with evidence of HT compared to those without HT. Previous studies have similarly shown that risk factors such as demographic characteristics, a history of essential hypertension, diabetes mellitus, and CVD are associated with an increased risk and severity of ICH (29, 30). A study conducted by the Multicenter Stroke Survey Group in the early 2000s revealed a significant four-fold increase in the risk of sICH following IV r-tPA treatment in diabetic patients. Furthermore, it was observed that there was a significant correlation between the increase in glucose level (per 50 mg/dL) and the occurrence of ICH (6).
A meta-analysis study conducted by Wen et al. revealed that the presence of diabetes in Chinese patients with AIS who received thrombolytic therapy was significantly associated with an increased risk of HT (31). The findings of our study are consistent with those of previous investigations.
Seet et al. conducted a study involving 212 patients with Acute AIS who received intravenous r-tPA. Among these patients, 14 had a history of warfarin use and were found to have a higher risk of sICH compared to other patients, despite having INR values below 1.7. Furthermore, patients treated with warfarin exhibited higher mortality rates and poorer recovery outcomes (32). A systematic review and meta-analysis of 19 studies involving 108,588 patients revealed a positive correlation between antiplatelet drug use and the occurrence of sICH. However, the differences in outcomes and mortality were not statistically significant (33). Khazaei et al. compared the occurrence of sICH and stroke severity in patients with a history of antiplatelet therapy. Their findings showed a higher prevalence of sICH and worse outcomes in these patients when treated with the standard dose of r-tPA (34). Our study found that most patients did not report a history of using antiplatelet or anticoagulant drugs. However, a significant association emerged between antiplatelet drug use and the occurrence of HT following intravenous r-tPA. These findings align with the results of previous studies on this topic.
Following the onset of AIS, circulating neutrophils rapidly migrate to the site of cerebral injury. Neutrophils are among the first cell types to infiltrate hypoxic tissue, a process that occurs within the initial hours after reperfusion. Neutrophils release Matrix Metalloproteinase-9, along with other inflammatory mediators and oxygen-free radicals, which have the potential to induce damage to the BBB and thereby contribute to ICH and augmented infarct size (35-37).
NLR, a laboratory biomarker, has been extensively examined in numerous studies. It has demonstrated its potential as a reliable predictor of outcomes, specifically in relation to the stroke-induced acute inflammatory response. Consistently, elevated NLR values have been linked to a poorer functional status among patients following AIS (38, 39).
In a study conducted by Im and Cañete (40) at a tertiary hospital in the Philippines from July 2018 to July 2019, 500 ischemic stroke patients were evaluated. Their findings revealed a significant association between leukocytosis, characterized by a mean white blood cell (WBC) count of 14.5 × 10³/μL, and HT. In a study led by Xie et al. (41), a total of 251 patients who received r-tPA treatment were examined. The findings revealed that patients with an NLR ≥ 3.322 faced a 3.492-fold increased risk of ICH, and those with an NLR ≥ 5.511 had a 3.024-fold increased risk of experiencing unfavorable outcomes. Individuals with unfavorable outcomes exhibited increased levels of leukocytes following r-tPA therapy, including leukocyte count [adjusted OR (aOR) 1.191 for HT and 1.184 for unfavorable outcomes], neutrophil count (aOR 1.215 and 1.214), and NLR (aOR 1.084 and 1.091). The study concluded that the NLR after r-tPA administration demonstrated the most robust association with HT and unfavorable outcomes.
In the current study, the average percentage of neutrophils after r-tPA injection was higher than before IV r-tPA (80.07% vs. 77.79%). Following cerebral ischemia, lymphocyte levels decrease in contrast to neutrophil levels, leading to an elevated NLR (42). The comparison between the NLR before and after r-tPA injection in our study underscores the importance of monitoring post-treatment inflammatory markers. While baseline NLR is not predictive of hemorrhage, the NLR measured after r-tPA administration provides valuable insight into the risk, with an AUC of 0.768. Incorporating post-treatment NLR into clinical assessments can improve the accuracy of hemorrhage predictions and guide risk-based treatment decisions in patients receiving r-tPA. A recent study by Maestrini et al. revealed that high neutrophil counts and NLR before thrombolysis in patients with AIS were associated with a higher risk of sICH and worse outcomes at three months (43). Xing et al. found that an NLR of 10.59 is associated with a higher likelihood of developing sICH (44). However, a study by Pektezel et al. concluded that NLR is not a reliable indicator of r-tPA effectiveness, r-tPA-induced HT, or long-term prognosis in the early hours following a stroke (45).
Our study has certain limitations. First, although data were prospectively collected through a multicenter stroke registry, the retrospective design introduces the potential for selection bias. Additionally, some patients did not undergo follow-up imaging due to significant changes in their condition, either showing substantial improvement or deterioration, which may have affected the study's primary outcome. Future research should involve a larger cohort of individuals who experience ICH after r-tPA therapy, with a focus on separating symptomatic and asymptomatic cases for more detailed analysis.
5.1. Conclusions
Consistent with previous research, our study demonstrates that patients with pre-existing conditions such as diabetes, hypertension, dyslipidemia, and those with a history of anticoagulant or antiplatelet therapy have an elevated risk of HT following thrombolysis for ischemic stroke. These findings highlight the importance of vigilant monitoring and individualized risk assessment, particularly for patients with these comorbidities. Understanding these risk factors is crucial for improving patient outcomes and guiding clinical decision-making in the management of ischemic stroke.
Our study also identified the NLR as a promising biomarker for predicting HT both at admission and after thrombolytic therapy. The NLR offers a cost-effective and accessible tool for risk assessment. As a simple, widely available, and inexpensive measure, the NLR could facilitate the early identification of patients at heightened risk for symptomatic hemorrhage after recanalization in AIS.
By exploring the complex interplay between patient characteristics, medication history, and laboratory parameters in relation to HT risk, this study provides valuable insights for clinicians and reinforces prior evidence on HT risk factors. These findings contribute to a more comprehensive understanding of HT risks and open avenues for future research into predictive biomarkers for ischemic stroke complications.