1. Background
Stroke has a high prevalence worldwide, with its incidence and prevalence increasing each year (1). It is the most severe neurological disorder globally, accounting for half of emergency hospital cases. Stroke is the second leading cause of death worldwide, and patients often experience various complications, including disability (2-5).
Stroke can occur at any age, but the causes differ between the young and the elderly. Many stroke causes are preventable through a healthy lifestyle (6-8). Contributing factors include poor lifestyle choices, high blood pressure, elevated blood lipids, alcohol and smoking, a diet high in sugar and salt, diabetes, chronic kidney disease, cardiovascular disease, genetic factors, and drug use (6, 8-13).
According to global burden of disease (GBD) statistics from 2010, individuals aged 20 to 64 account for 31% of strokes (13, 14). In 2010, approximately 16.9 million people globally experienced a stroke (14). Other studies report stroke prevalence ranging from 2% to 23%, with variations due to differences in stroke categorization, study methodologies, and follow-up durations (15-19).
Strokes are classified into two types: Ischemic and hemorrhagic. Ischemic strokes result from blood vessel blockage, while hemorrhagic strokes involve blood vessel rupture. Hemorrhagic strokes include intracerebral hemorrhage and subarachnoid hemorrhage (12, 20). Ischemic strokes are more common than hemorrhagic strokes and have a lower mortality rate, with 70 - 80% of all stroke-related deaths in 2004 attributed to ischemic strokes (20, 21).
Stroke diagnosis is based on patient history, radiological findings, and clinical examination by the attending physician, leading to appropriate treatment (22-24). Stroke treatment varies based on the type and patient needs, including both drug and invasive methods. Intravenous alteplase is a drug treatment administered as an emergency measure in the early hours of patient admission (25). Mechanical thrombectomy (MT) is another treatment for stroke, crucial for large vessel occlusion (LVO). Although MT offers significant therapeutic benefits, it may cause complications such as hematoma, vascular damage, cerebral edema, and reperfusion injury, similar to other invasive procedures (26, 27).
2. Objectives
Given the high prevalence of stroke, this study aims to determine the role of hyperdense signal length in the middle cerebral artery on the degree of disability in patients with arterial ischemic stroke and evidence of vascular involvement.
3. Methods
In this cross-sectional study conducted between 2022 and 2023, patients with stroke who were referred to the emergency department of Imam Hossein Hospital in Tehran province were included. Inclusion criteria were: A definitive diagnosis of stroke according to a neurologist's opinion; the presence of diagnostic documents for stroke, such as a CT scan; informed consent to participate in the study; accessibility to the patient, including having a mobile phone for the patient or their family; and undergoing MT. Exclusion criteria were: Non-cooperation or withdrawal of consent by the patient at any time during the research; loss of access to the patient for any reason, including changes to the mobile phone number; and development of any complication or crisis affecting the patient's disability, such as a new disease or trauma. The tools used included the demographic form and the Modified Rankin Scale (mRS).
3.1. Demographic Information Form
The questionnaire included items about the patient's age, gender, the time elapsed from the onset of symptoms to the hospital visit (in hours), the time to the appearance of thrombectomy symptoms (in hours), middle cerebral artery (MCA) density (in millimeters), transformation hemorrhage, and whether the patient received rtPA medication (yes or no).
3.2. Modified Rankin Scale
The mRS scale is used to assess disability in patients with acute stroke, evaluating their disability across six levels. A score of zero indicates no symptoms, while a score of five signifies severe disability. The validity and reliability of the mRS instrument have been confirmed in various studies (28-31).
In this study, patients who arrived at the hospital as emergencies were evaluated for clinical symptoms and history, and an emergency CT scan was performed. Based on clinical evidence and the attending physician's opinion, patients were assigned either to the drug treatment group or the thrombectomy treatment group. Since this study focused exclusively on patients who underwent MT, only those with this diagnosis were included, while others were excluded.
At the beginning of the patient's admission, the demographic form and the mRS tool were completed. The mRS tool was then administered again at discharge and three months after discharge. All procedures adhered to the guidelines of Shahid Beheshti University's research ethics committee, as specified in the ethics code IR.SBMU.MSP.REC.1402.344. Following data collection, the patients' information was entered into SPSS version 16 software for analysis.
4. Results
The results showed that the mean ± SD age of the patients was 57.79 ± 13.56 years. The time from symptom onset to hospital arrival was 3.38 ± 2.32 hours, and the time from symptom onset to thrombectomy was 4.54 ± 2.55) hours. The MCA density status was 22.53 ± 5.75 mm. Additionally, 81% of patients did not have hemorrhage, and 88.1% did not receive r-tPA.
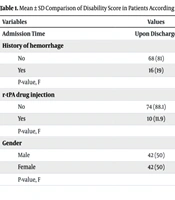
According to the findings in Table 1, the mRS score of patients with a history of hemorrhage was higher than that of other patients at all times during the study, and this difference was statistically significant (P < 0.05). Additionally, no significant relationship was observed between r-tPA drug injection and the mRS score of patients at any of the examined times (P > 0.05).
| Variables | Values | Modified Rankin Scale | ||
|---|---|---|---|---|
| Admission Time | Upon Discharge | 3 Months After Discharge | ||
| History of hemorrhage | ||||
| No | 68 (81) | 4.74 ± 0.47 | 3.58 ± 1.82 | 3.26 ± 2.23 |
| Yes | 16 (19) | 4.93 ± 0.25 | 5.43 ± 1.03 | 5.68 ± 0.79 |
| P-value, F | 0.000, 14.01 | 0.007, 7.67 | 0.000, 32.1 | |
| r-tPA drug injection | ||||
| No | 74 (88.1) | 4.78 ± 0.44 | 3.97 ± 1.9 | 3.78 ± 2.30 |
| Yes | 10 (11.9) | 4.80 ± 0.42 | 3.7 ± 1.33 | 3.30 ± 1.82 |
| P-value, F | 0.76, 0.09 | 0.12, 2.35 | 0.13, 2.28 | |
| Gender | ||||
| Male | 42 (50) | 4.76 ± 0.48 | 4.26 ± 1.98 | 4.30 ± 2.22 |
| Female | 42 (50) | 4.80 ± 0.40 | 3.61 ± 1.65 | 3.14 ± 2.14 |
| P-value, F | 0.66, 0.98 | 0.11, 1.01 | 0.01, 0.002 | |
a Values are expressed as mean ± SD or No. (%).
According to the findings, there was no relationship between the patient's age and the disability score at the time of admission (P = 0.11). However, at the time of discharge and 3 months after discharge, the level of disability was significantly related to the patient's age, with younger patients reporting less disability (Table 2).
| Variables | R | R Square | Adjusted R Square | Sum of Squares | Mean Square | F | Sig. |
|---|---|---|---|---|---|---|---|
| Admission time | 0.174 | 0.030 | 0.018 | 14817.751 | 182.935 | 2.543 | 0.11 |
| Upon discharge | 0.304 | 0.092 | 0.081 | 13870.735 | 169.155 | 8.352 | 0.005 |
| 3 months after discharge | 0.313 | 0.098 | 0.087 | 13873.724 | 168.094 | 8.923 | 0.004 |
According to the findings in Table 3, no significant relationship was observed between the time of assessing disability status and the duration from the onset of symptoms to the time of the patient's visit (P > 0.05).
| Variables | R | R Square | Adjusted R Square | Sum of Squares | Mean Square | F | Sig. |
|---|---|---|---|---|---|---|---|
| Admission time | 0.144 | 0.021 | 0.009 | 437.429 | 5.4 | 1.722 | 0.19 |
| Upon discharge | 0.147 | 0.022 | 0.010 | 440.595 | 5.373 | 1.808 | 0.18 |
| 3 months after discharge | 0.163 | 0.026 | 0.015 | 438.407 | 5.346 | 2.226 | 0.14 |
According to the findings in Table 4, no significant relationship was observed between the duration from the onset of symptoms until thrombectomy and the level of disability (P > 0.05).
| Variables | R | R Square | Adjusted R Square | Sum of Squares | Mean Square | F | Sig. |
|---|---|---|---|---|---|---|---|
| Admission time | 0.155 | 0.024 | 0.012 | 523.611 | 6.464 | 2.000 | 0.16 |
| Upon discharge | 0.160 | 0.026 | 0.014 | 526.917 | 6.426 | 2.155 | 0.14 |
| 3 months after discharge | 0.150 | 0.023 | 0.011 | 528.561 | 6.446 | 1.893 | 0.17 |
According to the findings in Table 5, a significant relationship was observed between the condition of MCA density and the level of disability in patients. As MCA density increased, so did the patients' disability, which was statistically significant (P < 0.05).
| Variable | R | R Square | Adjusted R Square | Sum of Squares | Mean Square | F | Sig. |
|---|---|---|---|---|---|---|---|
| Admission time | 0.248 | 0.061 | 0.050 | 10471.049 | 129.272 | 5.308 | 0.02 |
| Upon discharge | 0.219 | 0.048 | 0.036 | 10697.703 | 130.460 | 4.130 | 0.04 |
| 3 months after discharge | 0.288 | 0.083 | 0.072 | 10304.345 | 125.663 | 7.418 | 0.008 |
Abbreviation: MCA dence, dense middle cerebral artery.
According to the findings in Table 6, after the patient was admitted to the hospital and underwent thrombectomy, the patient's disability status was significantly reduced compared to the status at the time of admission (P = 0.000). The mean ± SD mRS score at the time of admission was 4.78 ± 0.44, while at the time of discharge it was 3.94 ± 1.84. Comparing the disability scores 3 months after discharge, the mean ± SD decreased from 4.78 ± 0.44 to 3.72 ± 2.25. This reduction compared to the score at the time of discharge was not statistically significant (P = 0.06).
In this study, the prevalence of disability was assessed using the mRS tool. The mean ± SD mRS score at the time of admission was 4.78 ± 0.44, at discharge it was 3.94 ± 1.84, and 3 months after discharge it was 3.72 ± 2.25.
Three months after discharge, the mRS scores for patients were as follows: 12 patients (14.3%) had a score of 0, 6 patients (7.1%) had a score of 1, 10 patients (11.9%) had a score of 2, 9 patients (10.7%) had a score of 3, 5 patients (6%) had a score of 4, 12 patients (14.3%) had a score of 5, and 30 patients (35.7%) had a score of 6.
| Variables | Paired Differences | t | Sig. (2-Tailed) | ||||
|---|---|---|---|---|---|---|---|
| Mean | Std. Deviation | Std. Error Mean | 95% Confidence Interval of the Difference | ||||
| Lower | Upper | ||||||
| Comparison of the disability score at the time of admission and discharge | 0.79518 | 1.62119 | 0.17795 | 0.44118 | 1.14918 | 4.469 | 0.000 |
| Comparison of the disability score at the time of discharge and 3 months after discharge | 0.21429 | 1.03052 | 0.11244 | -0.00935 | 0.43792 | 1.906 | 0.06 |
5. Discussion
Diseases related to the brain and nervous system can lead to various complications, including disability, for patients (32-35). Disability is a common complication that impacts all aspects of a patient's life (36, 37). Therefore, this study aimed to compare the role of hyperdense signal length in the middle cerebral artery on the degree of disability in patients with arterial ischemic stroke and large vessel involvement. Numerous studies have investigated the prevalence of disability in stroke patients, revealing differences in study methods, tools used, and patient demographics. This study will compare its findings with those of other studies to identify similarities and differences.
In a prospective cohort study by Ghandehari et al., which explored factors influencing disability in stroke patients, 122 patients (37.4%) had positive hemi hypoesthesia, 207 patients (62.6%) had negative hemi hypoesthesia, 45 patients (13.8%) had hemi anesthesia, and 284 patients (86.2%) had negative hemi anesthesia (38).
Connell et al. examined 70 stroke patients and found impaired tactile sensations in 7 - 53% of patients, impaired stereognosis in 31 - 89%, and impaired proprioception in 34 - 64% (39). Similarly, Mazidi et al. reported a mean ± SD functional independence score of 100.117 ± 23.69, indicating disability in these patients (40). The findings from these studies align with the present study's results regarding the presence of disability in stroke patients.
In the present study, the mean ± SD disability score for men was 4.30 ± 2.22 and for women was 3.14 ± 2.14, with this difference being statistically significant. Konduru et al. reported an initial mean mRS score of 3.48 and a final mean mRS score of 2.01 for men. For women, the initial mRS score was 2.1 and the final mRS score was 3.44 (41). The difference in mRS scores between this study and Konduru et al. (41) may be attributed to differences in treatment types. In this study, thrombectomy treatment was performed, whereas Konduru et al. used drug treatment, which may have influenced the disability status outcomes.
In this study, 3 months after the patient's discharge, the mRS scores were as follows: 12 patients (14.3%) had a score of zero, 6 patients (7.1%) had a score of one, 10 patients (11.9%) had a score of two, 9 patients (10.7%) had a score of three, 5 patients (6%) had a score of four, 12 patients (14.3%) had a score of five, and 30 patients (35.7%) had a score of six. In the study by Erler et al., which examined patients with upper extremity issues after ischemic stroke using the mRS questionnaire, the scores at 90 days were as follows: 73.2% of patients had a score of zero, 73.16% had a score of one, 73.21% had a score of two, 73.20% had a score of three, 73.12% had a score of four, and 73.2% had a score of five (42). Additionally, ElHabr et al. reported on acute ischemic stroke patients with the following mRS scores at 30 days: 64 patients (22.9%) had a score of zero, 57 patients (20.4%) had a score of one, 36 patients (12.9%) had a score of two, 46 patients (16.4%) had a score of three, 58 patients (20.7%) had a score of four, 15 patients (5.4%) had a score of five, and 4 patients (1.4%) had a score of six. For the 90-day mRS, the scores were: 75 patients (26.8%) had a score of zero, 59 patients (21.1%) had a score of one, 17 patients (6.1%) had a score of two, 46 patients (16.4%) had a score of three, 42 patients (15.0%) had a score of four, 19 patients (6.8%) had a score of five, and 22 patients (7.9%) had a score of six (43).
5.1. Conclusions
Considering that older age and higher MCA levels were associated with greater disability, it is necessary to implement preventive measures in this area.