1. Background
Schizophrenia is a heterogeneous and disabling mental disorder characterized by a wide array of symptoms, which can be broadly categorized into positive symptoms (such as hallucinations, delusions, and disorganized thinking), negative symptoms (including apathy, anhedonia, and social withdrawal), and cognitive impairments. Cognitive deficits are considered a core symptom, with nearly all patients (98%) showing a decline in cognitive functioning compared to their premorbid stage (1). These deficits are commonly observed in areas such as verbal fluency, processing speed, attention, working memory, executive functioning, and declarative verbal memory, all of which contribute to the poor functionality and burden of the disorder (1-5).
Interestingly, the severity of cognitive deficits in schizophrenia does not always correlate with the severity of positive and negative symptoms. Some patients exhibit severe cognitive impairments despite having mild positive and negative symptoms, while others may show pronounced psychotic symptoms but relatively preserved cognitive abilities (6). This dissociation underscores the complexity and multifaceted nature of cognitive disturbances in schizophrenia.
Moreover, individuals with schizophrenia are at a higher risk of developing metabolic disorders, such as diabetes, which have been identified as significant risk factors for cognitive impairments (7). The co-occurrence of these metabolic conditions can worsen cognitive deficits, contributing to a more debilitating clinical profile.
Despite advances in pharmacological and psychological treatments for schizophrenia, cognitive and functional deficits often persist, even when positive and negative symptoms improve (8). This poses a substantial challenge, as cognitive impairments are closely linked to poor functional outcomes and reduced quality of life for people with schizophrenia (9).
The factors underlying the persistence of cognitive and functional deficits in schizophrenia remain poorly understood (8). Identifying and understanding these factors is crucial for developing more effective treatment approaches and improving the overall prognosis for individuals with this complex disorder.
2. Objectives
The present study aims to address this gap in the literature by identifying the underlying factors related to the baseline cognitive state, as well as the changes in cognitive status and executive function following treatment in patients with schizophrenia. By elucidating these key determinants, the study seeks to provide valuable insights that can inform the development of targeted interventions, thereby enhancing the management of cognitive deficits in schizophrenia.
3. Methods
3.1. Study Design and Participants
This study utilized a short-term observational and longitudinal design to investigate the primary correlates of cognitive deficits in individuals diagnosed with schizophrenia. The study sample comprised 30 hospitalized patients (15 males and 15 females), aged between 18 and 65 years, recruited from the psychiatric inpatient unit of a tertiary care hospital. The diagnosis of schizophrenia was confirmed by two experienced psychiatrists using the structured clinical interview for DSM-5 (SCID-5), ensuring a standardized diagnostic process. Data were collected before hospitalization and after discharge over the course of one year.
Strict inclusion criteria were applied to minimize potential confounding effects of comorbidities and other factors that could influence cognitive functioning. Specifically, the inclusion criteria were:
(1) Patients were not in the acute phase of the illness,
(2) They had no concurrent psychiatric or neurological disorders,
(3) They had no history of head injury or recent infectious diseases, and
(4) They had not consumed alcohol or illicit drugs in the last six months.
To ensure the validity of the abstinence criterion, legal guardians verified participants' substance use history, and all participants completed substance screening tests on the first day of admission. This careful selection process allowed the researchers to focus on the primary cognitive deficits associated with schizophrenia, rather than those influenced by other confounding factors.
The research team collected comprehensive data, including demographic information (such as gender), anthropometric parameters (such as Body Mass Index, BMI), age at onset of initial symptoms, smoking status, and family history of psychiatric disorders. These variables were chosen to explore their potential influence on cognitive functioning and to identify any correlations that could inform future treatment strategies. Data collection was conducted through face-to-face interviews with patients and their legal guardians, ensuring accuracy and reliability.
The longitudinal design, with cognitive assessments conducted at both admission and discharge, enabled the researchers to examine changes in cognitive function over the course of hospitalization and treatment. This approach provided valuable insights into the dynamic nature of cognitive deficits in schizophrenia, potentially informing more effective intervention strategies for improving cognitive outcomes.
3.2. Measurements and Definitions
The Positive and Negative Syndrome Scale (PANSS), developed by Kay and Sevy in 1990, comprehensively evaluates schizophrenia symptoms through 30 questions, each answered on a five-point scale. The questionnaire consists of five subscales: Negative symptoms (8 questions), positive symptoms (6 questions), dissociation (7 questions), irritability symptoms (4 questions), and anxiety and depression (5 questions). Kay and Sevy identified two main factors, negative and positive syndromes, which account for 36.1% of the total variance in schizophrenia symptoms (10). In a study by Ghamari Givi et al. (2009) in Iran, the Cronbach's alpha for the PANSS was 0.77, indicating acceptable internal consistency (11).
To assess cognitive function, the Brief Assessment of Cognition in Schizophrenia (BACS) was used. Developed by Richard Keefe in 1999, the BACS evaluates cognitive domains in schizophrenia, including verbal memory, sequencing, motor tasks, fluency, and executive function (8). In a study by Mazhari et al., the Persian version of the BACS had a Cronbach's alpha of 0.74 and demonstrated significant correlations with standard neurocognitive subscales, confirming its reliability (12). The current study aimed to identify the main correlates of different cognitive domains and assess changes in cognitive status and executive function following in-hospital treatment.
3.3. Statistical Methods
The Kolmogorov-Smirnov test was applied to assess the normality of quantitative research variables. For quantitative and qualitative variables, the mean (standard deviation) and number (percentage) were reported. Demographic variables and their distribution among male and female participants were analyzed using independent t-tests and Pearson's chi-square tests.
Individual profile plots were generated for each cognitive variable to examine changes in cognitive status over time. To analyze these changes, the generalized estimating equations (GEE) model was employed, both with and without adjustments for demographic variables. The GEE model is particularly suitable for longitudinal data analysis, where repeated measurements are taken from the same subjects, and it accounts for the correlation between observations within the same subject, providing robust estimates of population-averaged effects. This method allows for flexibility in handling various response distributions (e.g., normal, binomial, Poisson) and can manage missing data under certain conditions (13).
All statistical analyses were conducted using R-Studio software, version 2023.06.1. The dgof (Discrete Goodness-of-Fit Tests) package was used for the Kolmogorov-Smirnov test, the stats package for independent t-tests and Pearson's chi-square tests, ggplot2 for drawing individual change graphs, and geepack (Generalized estimating equation package) for fitting the GEE model in R-Studio. A two-sided P-value of < 0.05 was considered statistically significant.
4. Results
The Kolmogorov-Smirnov test was used to assess the normality assumption of the quantitative variables, and the results indicated that the quantitative variables, including cognitive status, followed a normal distribution (P-value > 0.05). According to Table 1, the average age of women was 44.20, while the average age of men was 37.33; however, this difference was not statistically significant (P-value = 0.163). The average BMI of men was 22.74, and the average BMI of women was 25.72, but this difference was also not statistically significant (P-value = 0.183). The distribution of smokers varied significantly between men and women (P-value < 0.001), whereas the distribution of family history of neurological disease showed no significant difference between genders (P-value = 0.920).
| Variables | Female (n = 15) | Male (n = 15) | P-Value |
|---|---|---|---|
| Age | 42.20 (15.46) | 37.33 (10.26) | 0.163 |
| Age-first | 20.60 (5.66) | 18.60 (3.64) | 0.260 |
| BMI | 25.72 (7.69) | 22.74 (3.44) | 0.183 |
| Smoking | < 0.001 | ||
| No | 11 (73.3) | 2 (13.3) | |
| Yes | 4 (26.7) | 13 (86.7) | |
| Family history | 0.925 | ||
| No | 6 (40) | 5 (34) | |
| Yes | 9 (60) | 10 (66) |
Abbreviations: Age-first, age at the onset of initial symptoms; BMI, Body Mass Index.
a Categorical and continuous variables are expressed as mean (SD) and number (%), respectively.
b The P-value was assessed using chi-square tests for categorical variables and the independent t-test for continuous variables.
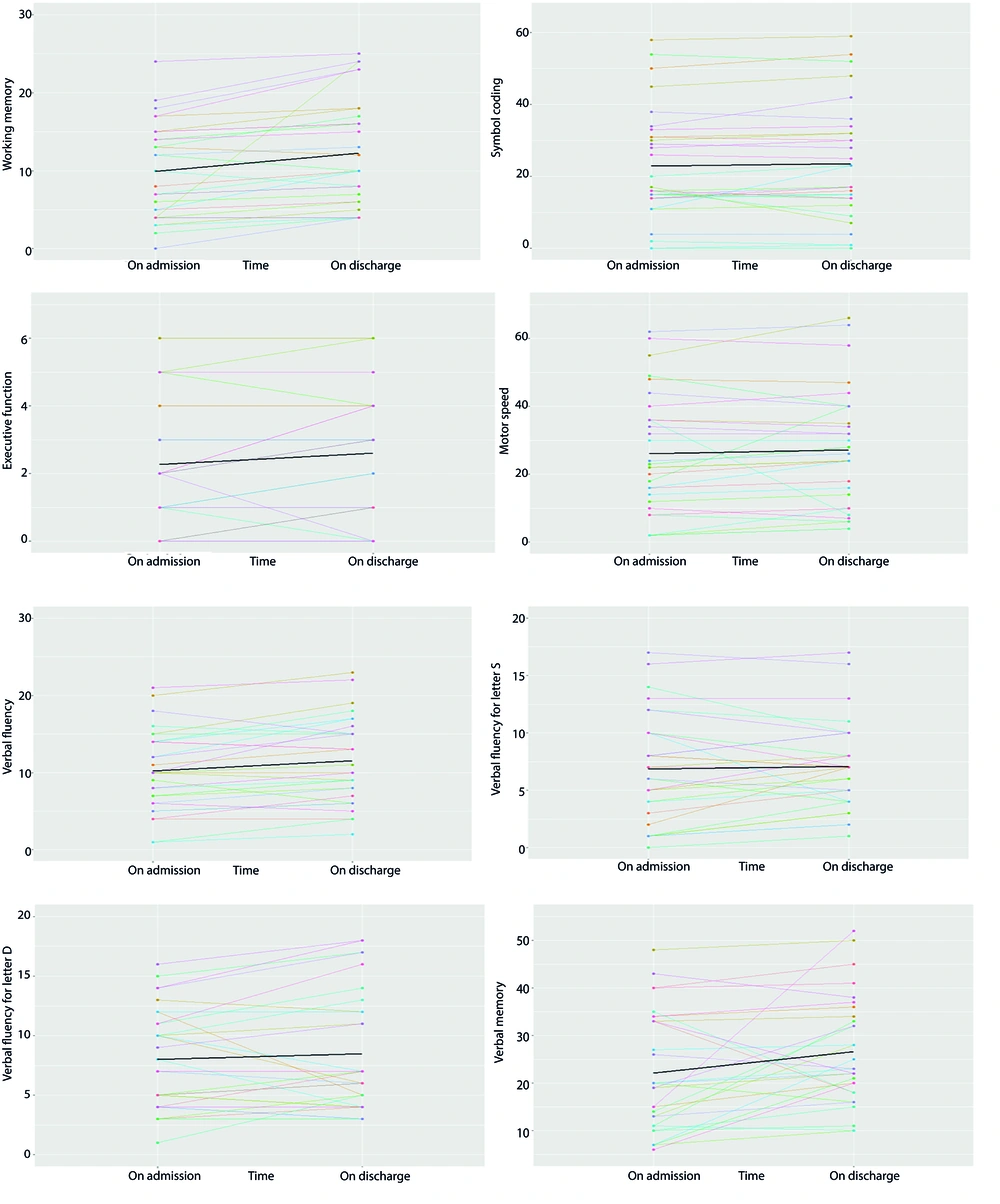
To analyze individual changes in cognitive state, an individual profile plot was created for each cognitive variable, including working memory, symbol coding, executive function, motor speed, verbal fluency, verbal fluency for letter S, verbal fluency for letter D, and verbal memory (Figure 1). These plots visually demonstrated individual changes in cognitive status. The graphs revealed that verbal memory, verbal fluency for letter D, verbal fluency, executive function, and working memory were generally lower at admission compared to discharge for most participants. The average trend line also indicated improvement in cognitive function upon discharge, highlighting the positive impact of hospitalization. However, verbal fluency for letter S, symbol coding, and motor speed showed minimal change between admission and discharge, with some measures even lower upon discharge.
A GEE model was applied to further investigate the data, focusing on time and cognitive variables as responses. The GEE model revealed that working memory, symbol coding, executive function, motor speed, verbal fluency, verbal fluency for letter S, verbal fluency for letter D, and verbal memory had higher averages at discharge compared to admission (Table 2). However, only verbal memory, working memory, and executive function showed statistically significant differences (β = 4.82, P = 0.031; β = 2.56, P < 0.001; β = 2.60, P = 0.022). For instance, the β coefficient for verbal memory indicated an average increase of 4.82 units from admission to discharge.
| Variables | On Admission | On Discharge | β | P-Value |
|---|---|---|---|---|
| PANSS | 84.00 (15.78) | 70.30 (12.48) | -13.79 | < 0.001 |
| Adverse reaction | 0.166 (0.379) | 0.466 (0.507) | 0.421 | 0.002 |
| Verbal memory and learning | 22.10 (12.37) | 26.60 (11.34) | 4.82 | 0.031 |
| Working memory | 9.90 (6.08) | 12.26 (6.90) | 2.56 | < 0.001 |
| Motor speed | 26.10 (17.74) | 27.16 (17.52) | 1.25 | 0.512 |
| Verbal fluency for letter S | 6.83 (4.72) | 7.10 (3.58) | 0.367 | 0.515 |
| Verbal fluency for letter D | 8.00 (4.20) | 8.50 (5.02) | 0.611 | 0.421 |
| Verbal fluency | 10.23 (5.20) | 11.50 (5.51) | 1.32 | <0.001 |
| Symbol coding | 22.90 (15.38) | 23.56 (16.10) | 0.652 | 0.411 |
| Executive function | 2.26 (1.86) | 2.60 (1.84) | 0.351 | 0.022 |
Abbreviation: PANSS, Positive and Negative Syndrome Scale.
a Continuous variables are expressed as mean (SD), respectively.
b The P-value indicates the significance of changes in each variable over time, independent of the effects of other variables.
To accurately assess the impact of demographic factors on cognitive status using the GEE model, the variables Age-First, Age, BMI, Sex, Family History, and Smoking were considered for adjustment (Table 3). The cognitive variables were used as responses in the analysis. The results showed that age had a significant negative effect on motor speed, verbal fluency for letter D, verbal fluency, and symbol coding (β = -0.720, P < 0.001; β = -0.107, P = 0.033; β = -0.141, P = 0.048; β = -0.504, P = 0.040). Conversely, Age-First had a positive effect on verbal fluency for letter D and verbal fluency for letter S (β = 0.649, P < 0.001; β = 0.503, P = 0.003).
| Variables | PANSS | Adverse Reaction | Verbal Memory | Working Memory | Motor Speed | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| β | P-Value | β | P-Value | β | P-Value | β | P-Value | β | P-Value | |
| Time | -13.70 | < 0.001 | 0.300 | < 0.001 | 4.50 | 0.023 | 2.36 | < 0.001 | 1.06 | 0.440 |
| Age-first | -0.211 | 0.743 | -0.022 | 0.151 | 0.178 | 0.629 | 0.407 | 0.171 | 1.13 | 0.104 |
| Age | 0.340 | 0.041 | 0.007 | 0.111 | -0.223 | 0.137 | -0.091 | 0.293 | -0.720 | < 0.001 |
| BMI | 0.028 | 0.942 | 0.013 | 0.218 | -0.320 | 0.292 | -0.086 | 0.720 | -0.094 | 0.838 |
| Sex | ||||||||||
| Female | Ref | Ref | Ref | Ref | Ref | Ref | Ref | Ref | Ref | Ref |
| Male | 16.42 | 0.008 | 0.218 | 0.286 | -9.05 | 0.172 | 2.67 | 0.216 | -1.17 | 0.850 |
| Family history | ||||||||||
| No | Ref | Ref | Ref | Ref | Ref | Ref | Ref | Ref | Ref | Ref |
| Yes | -2.87 | 0.520 | 0.067 | 0.550 | 1.64 | 0.645 | -2.63 | 0.241 | -6.75 | 0.132 |
| Smoking | ||||||||||
| No | Ref | Ref | Ref | Ref | Ref | Ref | Ref | Ref | Ref | Ref |
| Yes | -7.44 | 0.243 | -0.114 | 0.624 | 2.49 | 0.712 | -3.20 | 0.214 | -14.67 | 0.016 |
| Variables | VFD | Verbal Fluency | Symbol Coding | Executive Function | VFS | |||||
| β | P-Value | β | P-Value | β | P-Value | β | P-Value | β | P-Value | |
| Time | 0.500 | 0.295 | 1.26 | < 0.001 | 0.666 | 0.332 | 0.333 | 0.027 | 0.266 | 0.519 |
| Age-first | -0.649 | < 0.001 | 0.295 | 0.199 | -0.981 | 0.094 | -0.129 | 0.135 | -0.503 | 0.003 |
| Age | -0.107 | 0.033 | -0.141 | 0.048 | -0.504 | 0.040 | -0.020 | 0.337 | -0.069 | 0.158 |
| BMI | -0.008 | 0.952 | 0.063 | 0.709 | 0.019 | 0.969 | 0.016 | 0.737 | -0.007 | 0.962 |
| Sex | ||||||||||
| Female | Ref | Ref | Ref | Ref | Ref | Ref | Ref | Ref | Ref | Ref |
| Male | 0.745 | 0.594 | -3.68 | 0.023 | -19.18 | < 0.001 | -2.03 | 0.001 | -0.670 | 0.671 |
| Family history | ||||||||||
| No | Ref | Ref | Ref | Ref | Ref | Ref | Ref | Ref | Ref | Ref |
| Yes | -0.623 | 0.628 | -0.022 | 0.878 | -1.88 | 0.716 | 0.998 | 0.053 | -1.47 | 0.182 |
| Smoking | ||||||||||
| No | Ref | Ref | Ref | Ref | Ref | Ref | Ref | Ref | Ref | Ref |
| Yes | -1.59 | 0.316 | 2.14 | 0.284 | 8.46 | 0.246 | -1.46 | 0.039 | -0.040 | 0.918 |
Abbreviations: BMI, Body Mass Index; VFD, Verbal fluency for letter D; VFS, verbal fluency for letter S. β represents the change in the average value of cognitive components for every one-unit increase in each of the independent variables.
a Models are adjusted for age, BMI, Age-First, Sex, family history, and smoking.
Additionally, the GEE model revealed that men had higher PANSS scores than women (β = 16.42, P = 0.008), while verbal fluency, symbol coding, and executive function were lower in men (β = -3.68, P = 0.023; β = -19.18, P < 0.001; β = -2.03, P < 0.001). Smokers had lower executive function and motor speed than non-smokers (β = -1.46, P = 0.039; β = -14.67, P = 0.016). Furthermore, over time, individuals experienced significant improvements in verbal memory, working memory, verbal fluency, and executive function (β = 4.50, P = 0.023; β = 2.36, P < 0.001; β = 1.26, P < 0.001; β = 0.233, P = 0.027), indicating a general improvement in cognitive function following hospitalization and treatment.
5. Discussion
The present study aimed to investigate the changes in cognitive status of patients with schizophrenia and the factors influencing these changes over the course of hospitalization and treatment. Understanding the trajectory of cognitive deficits and their determinants is crucial, as cognitive impairments are a core feature of schizophrenia and contribute significantly to the overall burden of the disease. The findings provide valuable insights into the complex interplay between cognitive function, clinical symptoms, and demographic factors in individuals with schizophrenia.
Notably, the study results indicate significant improvements in specific cognitive domains, including verbal memory, working memory, and executive function, following hospitalization and standard treatment. These improvements suggest that a combination of inpatient care and pharmacological interventions can positively impact certain aspects of cognitive functioning in this patient population. The enhancements in these cognitive areas are particularly important, as deficits in verbal memory, working memory, and executive function are often associated with poor social and vocational outcomes in schizophrenia (2-5). Addressing these cognitive impairments in treatment plans could therefore be critical to improving long-term functional outcomes.
The findings align with previous research highlighting the potential of pharmacological agents, such as those targeting the glutamatergic and cholinergic systems and psychostimulants, to alleviate cognitive deficits in schizophrenia, though with modest and variable effects. For instance, Modafinil has been shown to enhance cognitive functions, including verbal memory, which is consistent with the improvements observed in this study. This reinforces the idea that targeted pharmacological interventions may play a role in improving cognitive outcomes, although individual responses can vary (14-23).
Demographic factors were also found to significantly influence cognitive status in schizophrenia. The study revealed that older age was associated with poorer performance on cognitive tasks, particularly in motor speed, verbal fluency, and symbol coding. This finding is consistent with existing literature suggesting that aging exacerbates cognitive decline, especially in individuals with pre-existing psychiatric conditions. Moreover, a later age of onset for the first symptoms of schizophrenia was linked to declines in verbal fluency, emphasizing the importance of comprehensive intervention strategies to mitigate long-term cognitive deficits (8). The study's findings on the limited improvement in verbal fluency following treatment with atypical antipsychotics are also consistent with previous reports (23).
Additionally, male patients and smokers exhibited lower cognitive function, particularly in verbal fluency, symbol coding, and executive function. The gender differences in cognitive function may be due to a combination of neurobiological differences, sociocultural factors, and healthcare disparities. Prior studies suggest that men may experience more severe cognitive impairments due to a combination of biological and psychosocial influences (24-26). Smoking was also found to negatively impact cognition, which aligns with previous research showing that smoking exacerbates cognitive deficits in schizophrenia. Potential mechanisms underlying this relationship include oxidative stress, neuroinflammation, and disruptions in neurotransmitter systems, indicating that smoking cessation may be a critical component of cognitive rehabilitation for patients with schizophrenia (27).
The longitudinal design of this study, along with the use of the GEE model, allowed for a thorough examination of cognitive changes over time while accounting for demographic and clinical variables. This approach provides a more nuanced understanding of the multifactorial nature of cognitive deficits in schizophrenia, revealing that while some cognitive domains improve with treatment, factors like age, gender, and smoking status continue to play significant roles (13).
In conclusion, this study underscores the potential for certain cognitive domains to improve with comprehensive inpatient treatment and standard pharmacological interventions in individuals with schizophrenia. However, the persistence of cognitive deficits, especially in relation to demographic factors such as age, gender, and smoking status, highlights the need for continued research and the development of targeted cognitive remediation strategies. By identifying the factors that influence cognitive function in schizophrenia, this study contributes to a growing body of knowledge aimed at enhancing overall functioning and quality of life for individuals affected by this complex and debilitating disorder.
5.1. Conclusions
The findings of the present study hold important clinical implications for managing cognitive deficits in individuals with schizophrenia. The observed improvements in verbal memory, working memory, and executive function following hospitalization and standard treatment suggest that a comprehensive, multifaceted approach to care can positively impact cognitive functioning in this patient population. This underscores the need to ensure access to high-quality inpatient services and evidence-based pharmacological interventions for individuals with schizophrenia. However, the poorer outcomes observed in cognitive domains like verbal fluency highlight the necessity for more extensive research and interventions to address disabling cognitive impairments, considering both pharmaceutical and non-pharmaceutical approaches.
Furthermore, the identification of demographic factors—such as age, gender, and smoking status—as key determinants of cognitive function provides valuable insights for developing targeted cognitive remediation strategies. The finding that older age is associated with poorer performance in motor speed, verbal fluency, and symbol coding suggests that age-specific cognitive rehabilitation programs may be beneficial, with a focus on enhancing these domains. Additionally, the observed gender differences in cognitive function call for tailored interventions that address the unique needs of male and female patients with schizophrenia.
The negative impact of smoking on cognitive function, particularly in verbal fluency, symbol coding, and executive function, emphasizes the importance of incorporating smoking cessation programs into the comprehensive management of schizophrenia. Addressing this modifiable risk factor may help mitigate the worsening of cognitive deficits and improve functional outcomes for these individuals.
By clarifying the complex interplay between cognitive function, clinical symptoms, and demographic factors in schizophrenia, this study provides a foundation for the development of personalized, multidimensional approaches to managing cognitive deficits. Integrating these findings into clinical practice could enhance the effectiveness of cognitive remediation strategies, improve overall functioning, and ultimately elevate the quality of life for individuals living with this challenging disorder.