1. Background
Migraine is a headache disorder considered among the top 10 specific causes of disability and is the third most prevalent disorder worldwide, affecting 14.7% of the global population (1, 2). In a study conducted in the USA among 162,576 participants, the prevalence of migraine was found to be 11.7%, with a higher prevalence in participants aged 30 - 39 years (3). Additionally, it has been reported that migraine is most common among Caucasians (4) and has an estimated annual cost of 9.2 billion dollars (5).
Migraine, as a chronic neurological disorder, is characterized by a unilateral, paroxysmal, pulsating headache with attacks lasting between 4 - 72 hours, usually accompanied by nausea, phonophobia, and photophobia. Migraine includes two major subtypes: (1) Migraine without aura (MO) and (2) Migraine with aura (MA), which constitutes about 30% of migraineurs. It should be noted that the exact pathological and neurological etiology of migraine remains controversial (1).
Numerous imaging studies on the brains of migraine patients suggest some structural changes compared to healthy populations (6-12). For instance, in a cross-sectional study of structural brain lesions and headaches in 780 elderly participants, Kurth et al. demonstrated that any history of chronic severe headaches was correlated with higher risks of increased volumes of white matter hyperintensity (6). In another study, Mathur et al. showed that migraine could reduce neural activities associated with cognition in brain regions related to cognitive processing (7). In a paper exploring migraine, cognition, and brain structure, Nichole Schmitz et al. reported decreased parietal and frontal lobe gray matter density in migraineurs compared to the healthy/control group. They also highlighted a significant correlation between delayed response time and reduced frontal lobe gray matter density, suggesting the possible effect of migraine on the cognitive functions of the frontal lobe (8).
According to these structural changes in the brains of migraine patients, brain functions, particularly cognitive functions, should be assessed. Studies investigating the relationship between migraine and cognition have shown conflicting results. Some studies suggest that migraine could lead to poorer cognitive functions (13-16). Conversely, other studies suggest that migraine does not affect cognition (17-19). However, some studies indicate that migraine may enhance cognitive functions (20, 21).
Additionally, some of these studies also present discrepant results regarding the effects of MA and MO on cognitive function (13, 15, 16, 18, 20, 21). De Araujo et al. suggested more adverse changes in cognition in patients with MA (15), whereas Pellegrino Baena et al. suggested that MO, but not MA, could worsen cognition (16). Interestingly, some studies show better cognitive function in patients with MA or MO compared to healthy subjects (20, 21). Given that the frontal lobe plays an integral role in cognition (22) and its migraine-induced structural changes observed in previous imaging studies (8, 11), the relationship between migraine and the cognitive functions of the frontal lobe should be assessed. A simple tool to evaluate the cognitive function of the frontal lobe is the frontal assessment battery (FAB) test (23), a bedside test designed to assess frontal lobe functions and dysexecutive syndrome. The overall score of the FAB can determine the severity of the dysexecutive syndrome and might evaluate executive dysfunction (23).
The FAB has been demonstrated as a reliable instrument for detecting cognitive impairment in diseases such as amyotrophic lateral sclerosis (ALS), dementia, and Parkinson’s disease (PD) (24-27). Interestingly, Terada et al. suggested that in Alzheimer’s disease (AD) patients, the results of the FAB are more correlated with neurodegenerative changes seen in the frontal lobe than with amyloid-beta (Aβ) deposition and pathology (28). Furthermore, the FAB is suggested to be a determining tool for detecting frontal lobe lesions in disorders such as frontal lobe tumors and frontal cortex stroke (29, 30).
Studies that have evaluated cognition using the FAB in migraine patients are limited. Deodato et al. observed that the FAB results showed a notable reduction in patients with MO compared to healthy controls (31). Additionally, Le Pira et al., by comparing the FAB results in patients with MA and the control group, observed a considerable decline in FAB scores in patients with MA (13).
2. Objectives
Given the conflicting results in previous studies regarding the relationship between migraine and cognition, as mentioned above, and the abnormal structural findings in previous imaging studies found in the brains of migraineurs, particularly in their frontal lobe (6-12), the relationship between migraine and cognition should be assessed from different perspectives and cognitive tests. This approach will lead to a better understanding of the cognitive effects of migraine. Considering the limited number of studies using the FAB to evaluate cognitive functions in migraineurs and the discrepant findings on the effects of MA and MO on cognition, the present study employs the FAB test to evaluate the association between migraine and cognitive function, especially in the frontal lobe.
3. Methods
3.1. Study Design, Setting, and Participants
This prospective cross-sectional study included 48 migraine patients and 48 healthy subjects. The study was conducted in accordance with the STROBE guidelines, based on data collected between June and November 2022 in the neurology department of Tehran Imam Khomeini Hospital clinics (Appendix 1 in Supplementary File).
Migraine patients were initially selected through consecutive sampling from those referred to the clinic. All included migraine patients were over 18 years of age, had not experienced any headaches 25 hours prior to the interview, and were diagnosed based on the international classification of headache disorders, 3rd edition (beta version) (ICHD-3 beta) criteria. Migraine patients with any history of central or peripheral nervous system disorders, diagnosed psychological disorders based on clinical or medical records, or use of narcotics or hallucinogenic drugs were excluded.
Control subjects were gathered using frequency and stratified matching methods to ensure comparability across potential confounding variables such as age, sex, years of education, Body Mass Index (BMI), smoking status, and drinking history. All control subjects were healthy individuals over 18 years of age, chosen from patients' families and healthcare workers. Those with any history of prior migraine diagnosis, moderate to severe headaches, frequent headaches, central or peripheral nervous system disorders, diagnosed psychological disorders based on clinical or medical records, or use of narcotics or hallucinogenic drugs were excluded. All participants in this study were native Farsi speakers.
3.2. Ethical Considerations
This project was approved by the ethics committee of the Tehran University of Medical Sciences and was found to be in accordance with the ethical principles and the national norms and standards for conducting medical research in Iran (approval ID: IR.TUMS.IKHC.REC.1401.142). All participants provided informed verbal and written consent.
3.3. Covariates and Measurements
This study evaluated the FAB results of the study groups and independent variables. These variables consist of two groups:
3.3.1. Demographic Variables (Non-migraine-Specific Variables)
(1) Sex: Male or female
(2) Age: Years
(3) Smoking: A positive history was defined as at least one year of continuous smoking during their life (32) or a history of smoking for at least one month in the previous year of the interview (33).
(4) Drinking: History of consuming alcohol every week.
(5) Education: Total years of education of each participant.
(6) The BMI: Calculated based on standard criteria (34).
3.3.2. Migraine-Specific Variables
(1) Duration of the disease: The period between the diagnosis of the disease and our interview.
(2) Frequency of the disease: Number of days in a month during which participants had headaches.
(3) Severity of headaches: Evaluated based on a self-reported numerical pain rating score (35) between 0 (no pain) and 10 (worst imaginable pain).
(4) History of any preventive drug usage.
Frequency and stratified matching methods were applied to select the control group based on the demographic variables mentioned above to control the potential confounding effect of these variables. To further assess the potential confounding effect, demographic variables were compared between the migraineurs and the control group using statistical tests to confirm that there were no significant differences between these two groups.
In this study, the primary outcome was the comparison of the FAB results between all migraine patients and the control group, as well as the comparison of the FAB results based on demographic and migraine-specific variables (as mentioned above). However, some studies comparing cognition levels between patients with MA, MO, and healthy subjects have reported discrepant results, as mentioned earlier (13, 15, 16, 18, 20, 21). Therefore, we decided to add an alternative (accessory) objective, in which patients with MA, MO, and the control group were compared and analyzed for demographic variables and the FAB results. MA and MO were diagnosed based on ICHD-3 beta.
3.4. The Frontal Assessment Battery
The FAB is a scoring test with a maximum score of 18 and a minimum score of 0, designed to evaluate frontal lobe dysfunction. It is divided into six subsets based on six different frontal lobe cognitive functions: (1) Conceptualization, (2) mental flexibility, (3) motor programming, (4) sensitivity to interference, (5) inhibitory control, and (6) environmental autonomy. Each subset is scored from 0 to 3 based on its criteria. The test has demonstrated good interrater reliability (k = 0.87, P < 0.001), internal consistency (Cronbach’s coefficient alpha was 0.78%), and discriminant validity, with 89.1% of cases correctly identified in discriminant analysis of patients and controls (23).
The Persian-translated version of the FAB test has been validated in healthy individuals and patients with Parkinson's disease, demonstrating its reliability as a tool for assessing frontal lobe functions and its utility in evaluating cognitive decline and executive functions in the Iranian population (24). Following a thorough history taking, a general practitioner, trained by a neurologist in the standardized administration and scoring of the Persian-translated FAB test, administered the Persian-translated version of the FAB test during participants’ clinic visits.
3.5. Sample Size Calculation and Power Analysis
The G*Power software was utilized to perform the sample size calculation. Based on previous research, the effect size (Cohen’s d) was set at 0.58, which represents a moderate-to-large effect (14). Furthermore, the significance level (α) and statistical power (1-β) were set at 0.05 and 80%, respectively. Additionally, an equal allocation ratio (1:1) was used. This analysis indicated that a minimum of 96 participants (48 per group) were needed.
3.6. Statistical Analysis
This study used SPSS version 18 (Armonk, NY: IBM Corp) to perform the statistical analyses. The Kolmogorov-Smirnov z-test was used to assess the normal distribution of quantitative variables. After matching, statistical tests were performed to ensure that there were no significant differences between the migraine and control groups for demographic variables. Categorical variables such as sex, smoking, and drinking status were compared using the chi-squared test, while continuous variables such as age, BMI, and years of education were compared using the Mann-Whitney U test.
The statistical tests used for investigating the relationship between variables are as follows: The Spearman rank-order correlation coefficient was utilized for comparing quantitative variables where the data did not have a normal distribution. The Mann-Whitney U test and Kruskal-Wallis test were used for comparing qualitative variables with quantitative variables with non-normally distributed data. For categorical data, the chi-squared test was utilized for the comparison of qualitative variables. The results are presented as follows: For quantitative variables, we used mean ± standard deviation (STD), and median [interquartile range (IQR)] and for qualitative variables frequency (percentage) were used. All reported probability values were two-tailed, and a P-value < 0.05 was considered statistically significant.
4. Results
Of the 96 participants included in this cross-sectional study, 48 were healthy subjects (11 males, 37 females) with a median age of 40.50 years and an IQR of [18.75]. The remaining 48 were migraine patients (9 males, 39 females) with a median [IQR] age of 38.00 [13.00], of which 10 were diagnosed with MA and 38 with MO. The median [IQR] years of education for all participants, the control group, and all migraine patients were 16.00 [6.00], 16.00 [6.00], and 13.50 [4.75] years, respectively. Except for age, there were no significant differences between any of the non-migraine-specific variables (P-value > 0.05). Additionally, there were no significant differences in age between the control group and all migraine patients, nor between the control group and patients with MO (P-value > 0.05). However, there was a significant difference in age between the control group and patients with MA (P-value < 0.001), as well as between patients with Migraine with Aura and those with MO (P-value < 0.001). Other demographic and clinical characteristics of all participants are shown in Table 1.
| Variables | All Participants (N = 96) | Control (N = 48) | Migraine (N = 48) | MO (N = 38) | MA (N = 10) | P-Value Between Migraine and Control Group | P-Value Between Control, Migraine with and Without Aura |
|---|---|---|---|---|---|---|---|
| Sex | 0.61 b | 0.876 b | |||||
| Female | 76 (79.2) | 37 (77.1) | 39 (81.3) | 31 (81.6) | 8 (80.0) | ||
| Male | 20 (20.8) | 11 (22.9) | 9 (18.8) | 7 (18.4) | 2 (20.0) | ||
| Age | 0.30 c | 0.01 d,e | |||||
| Mean ± STD | 39.55 ± 11.51 | 41.02 ± 12.04 | 38.08 ± 10.88 | 40.36 ± 10.43 | 29.40 ± 8.07 | ||
| Median [IQR] | 40.00 [15.75] | 40.50 [18.75] | 38.00 [13.00] | 40.50 [10.00] | 27.50 [14.00] | ||
| Education | 0.40 c | 0.32 e | |||||
| Mean ± STD | 14.07 ± 4.48 | 14.37 ± 4.28 | 13.77 ± 4.71 | 13.28 ± 4.74 | 15.60 ± 4.32 | ||
| Median [IQR] | 16.00 [6.00] | 16.00 [6.00] | 13.50 [4.75] | 12.00 [4.00] | 16.00 [6.00] | ||
| BMI | 0.40 c | 0.59 e | |||||
| Mean ± STD | 25.60 ± 4.82 | 26.15 ± 5.50 | 25.06 ± 4.02 | 25.28 ± 4.35 | 24.21 ± 2.34 | ||
| Median [IQR] | 24.92 [5.18] | 25.47 [6.11] | 24.68 [4.57] | 24.88 [5.47] | 24.50 [2.82] | ||
| Alcohol | 0.18 b | 0.09 b | |||||
| Non-drinker | 86 (89.6) | 45 (93.8) | 41 (85.4) | 31 (81.6) | 10 (100.0) | ||
| Drinker | 10 (10.4) | 3 (6.3) | 7 (14.6) | 7 (18.4) | 0 (0.0) | ||
| Smoking | 1.00 b | 0.28 b | |||||
| Non-smoker | 80 (83.3) | 40 (83.3) | 40 (83.3) | 30 (78.9) | 10 (100.0) | ||
| Smoker | 16 (16.7) | 8 (16.7) | 8 (16.7) | 8 (21.1) | 0 (0.0) |
Abbreviations: MO, migraine without aura; MA, migraine with aura; STD, standard deviation; IQR, interquartile range; BMI, Body Mass Index.
a Values are expressed as No. (%) unless otherwise indicated.
b Chi-square test.
c Mann-Whitney test.
dStatistically significant.
e Kruskal-Wallis test.
All quantitative variables, including the FAB results, age, education, and BMI, were distributed non-normally. The FAB results in all migraine patients (median [IQR] FAB score: 15.00 [4.00]) were significantly worse than those in the control group 9median [IQR] FAB score: 16.00 [3.75]) (P-value = 0.04). Moreover, the performance of patients with MO (median [IQR] FAB score: 14.00 [5.00]) in the FAB results was significantly lower than that of the control group (P-value = 0.01). However, the differences in the FAB results between patients with MA (median [IQR] FAB score: 15.50 [1.50]) and the control group (P-value = 0.82), as well as between patients with MA and MO (P-value = 0.09), were not of significant importance (Table 2).
| Variables | Total | FAB | FAB | P-Value b |
|---|---|---|---|---|
| All participants | 96 (100) | 14.43 ± 2.94 | 15.00 [4.00] | |
| Groups between all participants | 0.04 c | |||
| Control/healthy | 48 (50) | 14.95 ± 2.79 | 16.00 [3.75] | |
| All migraine patients | 48 (50) | 13.91 ± 3.03 | 15.00 [4.00] | |
| Groups between all participants | 0.03 d | |||
| Control/healthy | 48 (50) | 14.95 ± 2.79 | 16.00 [3.75] | |
| MO | 38 (39.6) | 13.50 ± 3.21 | 14.00 [5.00] | |
| MA | 10 (10.4) | 15.50 ± 1.43 | 15.50 [1.50] |
Abbreviations: FAB, frontal assessment battery; STD, standard deviation; IQR, interquartile range.
a Values are expressed as No. (%) or mean ± STD or (median [IQR]).
b Statistically significant.
c Mann-Whitney test.
d Kruskal-Wallis test.
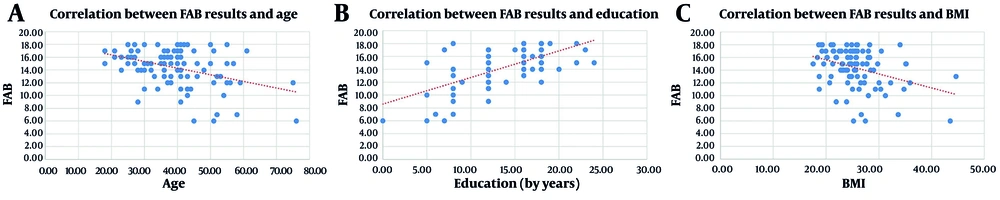
Regarding the numerical variables, there was a moderate statistically significant positive correlation between the FAB results and years of education (correlation coefficient = 0.54, R2 linear = 0.395, P-value < 0.001) and a mild statistically significant negative correlation between the FAB results and age (correlation coefficient = -0.32, R2 linear = 0.149, P-value < 0.001) and BMI (correlation coefficient = -0.35, R2 linear = 0.152, P-value < 0.001) (Figure 1).
Scatter plots showing the correlation between frontal assessment battery (FAB) results and age (A), education (B), and Body Mass Index (BMI) (C). The BMI, with trendlines. A positive moderate correlation was observed between FAB results and education (correlation coefficient = 0.54, P-value < 0.001), while negative mild correlations were found with age (correlation coefficient = -0.32, P-value < 0.001) and Body Mass Index (BMI) (correlation coefficient = -0.35, P-value < 0.001).
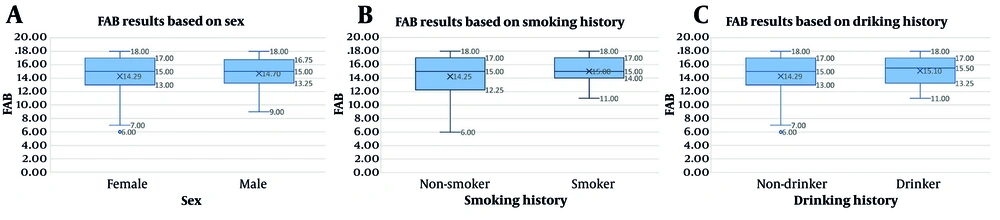
As for qualitative variables, the differences in FAB results between males (median [IQR] FAB score: 15.00 [3.50]) and females (median [IQR] FAB score: 15.00 [4.00]) were not statistically significant (P-value = 0.87). Regarding drinking, there were no significant differences in FAB results between drinkers (median [IQR] FAB score: 15.50 [3.75]) and non-drinkers (median [IQR] FAB score: 15.00 [4.00]) (P-value = 0.49). Furthermore, smokers with a median [IQR] FAB score of 15.00 [3.00] showed no significant differences compared to non-smokers (median [IQR] FAB score: 15.00 [4.00]) (P-value = 0.64) (Figure 2).
Box plots comparing frontal assessment battery (FAB) results based on sex (A), smoking history (B), and drinking history (C). Median FAB scores did not significantly differ between males and females (P-value = 0.87), smokers and non-smokers (P-value = 0.64), or drinkers and non-drinkers (P-value = 0.49).
Additionally, there were no significant differences in the FAB results based on preventive drug usage and the duration, frequency, and severity of the disease. The FAB results based on each migraine-specific variable are shown in Table 3.
| Variables | Migraine Patients (N = 48) | FAB | FAB | P-Value Between All Migraine Patients |
|---|---|---|---|---|
| Duration of disease (y) | 0.64 b | |||
| Under 1 | 13 (27.1) | 14.23 ± 2.71 | 15.00 [4.50] | |
| 1 to 5 | 16 (33.3) | 14.31 ± 3.23 | 15.00 [3.75] | |
| 5 to 10 | 8 (16.7) | 12.75 ± 3.61 | 13.00 [4.00] | |
| More than 10 | 11 (22.9) | 13.81 ± 2.82 | 15.00 [3.00] | |
| Days of headaches in a month (d) | 0.52 b | |||
| 1 - 10 | 29 (60.4) | 13.96 ± 3.71 | 15.00 [6.00] | |
| 11 - 20 | 11 (22.9) | 13.81 ± 1.88 | 14.00 [3.00] | |
| 21 - 30 | 8 (16.7) | 13.87 ± 1.12 | 14.00 [2.00] | |
| Severity of headaches | 0.97 b | |||
| Mild (1 - 3 scores) | 4 (8.3) | 14.00 ± 4.24 | 14.50 [8.00] | |
| Moderate (4 - 6 scores) | 13 (27.1) | 13.15 ± 4.33 | 15.00 [7.50] | |
| Severe (7 - 10 scores) | 31 (64.6) | 14.22 ± 2.17 | 14.00 [3.00] | |
| Preventive medication use | 0.73 c | |||
| Using treatment | 32 (66.7) | 13.90 ± 3.31 | 15.00 [4.00] | |
| Not using treatment | 16 (33.3) | 13.93 ± 2.46 | 14.00 [4.00] |
Abbreviations: FAB, frontal assessment battery; STD, standard deviation; IQR, interquartile range.
a Values are expressed as No. (%) or mean ± STD or (median [IQR]).
b Kruskal-Wallis test
c Mann-Whitney test
5. Discussion
This study aimed to evaluate the relationship between migraine and cognition using the FAB test. In the present study, the FAB results in all migraine patients were significantly worse than those in the control group. Additionally, the performance of patients with MO in the FAB results was considerably lower than that of the control group, suggesting worse cognitive function in all migraine patients, and specifically in patients with MO, compared to the control group. However, the differences in the FAB results between patients with MA and the control group were not significantly important. Also, in our study, the FAB results in patients with MA were higher than in patients with MO, but these results were not considerably important.
The differences in age between patients with MA and MO, and between patients with MA and healthy subjects, were significant. Thus, age could be considered a confounding factor in our study. However, the differences in age between all migraine patients and healthy subjects, and between patients with MO and healthy subjects, were not statistically significant. Therefore, the comparison of FAB results between patients with migraine and healthy subjects was not affected, and age had no confounding effect on our primary objective. Furthermore, it should be mentioned that age only had a weak negative correlation with the FAB results (Figure 1).
Studies investigating the relationship between migraine and cognition have shown conflicting results. Some studies suggest that migraine could lead to poorer cognitive functions. For instance, in a survey of 44 migraine patients and 16 control subjects, it was demonstrated that migraineurs generally had inferior results in cognitive tests; also, patients with MA performed worse in the FAB than patients with MO (13). In a meta-analysis of 17 studies on migraine and cognitive deficits, Braganza et al. suggested that migraine could have a negative, moderate effect on spatial cognition, executive function, immediate and delayed memory, and complex attention (14). Furthermore, de Araujo et al., in a systematic review of 23 studies on cognition, migraine, and cognitive impairment, proposed that migraine might increase the risk of cognitive impairment, with patients with MA displaying more cognitive changes (15). Additionally, Pellegrino Baena et al., in a cross-sectional analysis of the association between cognitive function and migraine among 4208 participants of the Brazilian Longitudinal Study of Adult Health, ELSA-Brasil, observed that migraineurs in general and patients with MO had poorer performance in cognitive tests; however, these results were not observed in patients with MA (16).
On the other hand, some studies suggest that migraine does not affect cognition. In a prospective cohort study, Rist et al. found that among 1170 participants (167 had migraine) of the epidemiology of vascular ageing study, using nine different tests, including the mini-mental state examination (MMSE), there was no greater cognitive decline in migraine patients compared to healthy participants (17). Also, in a blinded study on four different cognitive tests, Pearson et al. found that patients with both MA and MO did not differ significantly from matched controls (18).
However, other studies suggest that migraines may enhance cognitive functions. In a survey of 21 patients with MO and 21 healthy participants, Baschi et al. observed that MO was associated with better performances in learning and visuospatial memory (20). In a cross-sectional analysis of 6708 participants of the Rotterdam study, Wen et al. showed that patients with migraine, and especially those with MA, had better results in the MMSE, letter-digit substitution test, 15-word learning test, Stroop test, verbal fluency test, and Purdue pegboard test than healthy participants (21).
Though it is challenging to reconcile these conflicting results, the differences in the relationship between migraine and cognition could be explained by considering various factors. These include the different tests used for evaluating cognition and cognitive function, which are designed to assess different domains of understanding. Additionally, the populations from which participants are chosen in different studies, such as clinical populations or community samples, could influence the outcome of a survey. For example, Braganza et al., in their meta-analysis, showed that migraineurs recruited from clinical settings, such as neurology clinics, tend to present with more neuropsychological deficits than those chosen from the community (14).
Furthermore, factors such as different sample sizes, ethnic populations, languages, and socioeconomic statuses of participants could contribute to these inconsistent results. It should also be noted that the adverse effects of migraine on cognition observed in our study could align with the abnormal structural changes found in previous imaging studies (6-12). Altogether, our results could support the use of cognitive function assessment tools, including the FAB, to identify probable cognitive dysfunctions in patients with migraine, particularly those with MO. These results could also be beneficial for the early identification of possible changes in these patients in the future.
The results of our study suggest that differences in sex, drinking, and smoking status have no effects on the FAB results; therefore, we did not find any relationship between these variables and cognition (Figure 2).
Regarding the relationship between years of education, age, BMI, and cognition, our study found a moderate positive correlation between years of education and a mild negative correlation between age and BMI with the FAB results, and thus cognition (Figure 1). In a similar study, Mulholland et al. demonstrated that aging could reduce gray matter density and cognition (36). Additionally, in a cross-sectional study, Mumme et al. observed that younger age and having a university education were associated with better global cognitive function (37). Furthermore, Matallana et al. investigated the relationship between cognition and education, suggesting that more years of education correlate with better performance in the MMSE results (38).
As for BMI, studies show conflicting results. For example, Lynch et al. reported that a slower rate of cognitive decline with age was observed in subjects with a BMI ≥ 27.5 kg/m2 (39). However, Mwamburi and Qiu suggested that a higher BMI was associated with lower verbal IQ (40).
Another interesting result from our study was that there were no differences in the FAB results and cognition based on migraine-specific variables. In this study, differences in the frequency of migraine headaches did not cause changes in cognition; an increase in the disease duration among migraineurs did not cause differences in the FAB results. Consistent with our findings, Rist et al. observed that after 4 - 5 years of follow-up, migraine did not cause faster cognitive decline in patients (17). However, in another study, Zhao et al. showed that increases in the duration of the disease are related to progressive brain damage in regions associated with cognition and pain processing (41).
In our study, the severity of migraine headaches did not cause more cognitive decline. Inconsistent to our findings, Kurth et al. observed that the severity of any headache could increase the volume of white matter hyperintensities (6). The history of preventive drug usage did not improve the FAB results, and therefore cognitive function. Inconsistent with our results, Borsook et al. suggested that as the insula plays a vital role in cognitive function, migraine treatments can activate and affect insular cortex function and structure (42).
5.1. Conclusions
The present study suggests that a history of migraine, especially MO, significantly reduces the FAB results, thereby indicating worse cognitive function in patients with migraine compared to healthy populations. However, these results are not observed in patients with MA, and we cannot specify the relationship between MA and cognition, nor its differences with MO. Additionally, it should be noted that the FAB results and cognition could have a moderate positive correlation with years of education and a mild negative correlation with age and BMI. Furthermore, this study found no significant differences in the FAB results and cognition based on Duration of the disease, Frequency of the disease, severity of headaches, and the history of any preventive drug use.
5.2. Limitations
The cross-sectional method used in our study could limit the precise determination of the effect migraine has on FAB results and cognition. Furthermore, the limited sample size restricts a thorough evaluation of FAB results among migraine patients and the control group; the low sample size also limits the comparison between patients with MA, patients with MO, and healthy subjects. This study was conducted in the neurology department of Tehran Imam Khomeini Hospital clinics, which could result in socioeconomic and sampling biases. Additionally, due to financial constraints, we were unable to perform imaging evaluations of the study population.
5.3. Suggestions
Further studies are needed, as the cognitive changes in migraine patients can significantly influence their quality of life. For future research, multicenter longitudinal studies with larger patient populations could provide a more precise understanding of the cognitive effects of migraine and its subtypes. Additionally, the use of imaging modalities can help determine the specific brain regions affected by migraine and enable researchers to identify the mechanisms by which migraine impacts cognition. Furthermore, the utilization of a broader range of cognitive tests could assist researchers in evaluating the exact relationship between migraine and cognition.