1. Background
Inflammatory myopathies (IMs) are a heterogeneous group of inflammatory diseases that affect skeletal muscle, with or without the involvement of other organs, and are characteristically defined as myositis (1). Most inflammatory myositis cases are autoimmune in nature and are associated with chronic inflammation, immune dysregulation, and factors triggered by genetic or environmental influences (2, 3). Precise diagnosis is critical, as IMs are potentially treatable myopathies (4). Based on clinical and histopathological characteristics, three main subgroups of IMs have been defined: Polymyositis (PM), dermatomyositis (DM), and inclusion body myositis (IBM) (1). Recently, other IMs added to this subset include immune-mediated necrotizing myopathy (IMNM), overlap myositis (OM), and antisynthetase syndrome with lung involvement (2, 5). These myositis syndromes have been linked to autoimmune antibodies called myositis-specific antibodies (MSA) and myositis-associated antibodies (MAA). Hence, nonspecific inflammatory myopathies (NSIMs) are diagnosed when inflammatory markers and MSAs are not tested (3).
One of the common diagnostic tools for IMs is muscle biopsy. Additionally, the MSA panel is essential to aid muscle biopsy in subclassification. Dermatomyositis in muscle biopsy is characterized by the infiltration of lymphocytes into non-necrotic muscle fibers, which are subsequently replaced by macrophages (6, 7). Another specific and distinguishing feature of DM is perifascicular pathology (PFP), which includes perifascicular atrophy or necrosis (6). On the other hand, rimmed vacuoles are considered a hallmark feature of IBM (6, 7). The absence of inflammatory infiltrate and some of these characteristic features does not exclude the diagnosis of IMs. Therefore, major histocompatibility complex class I (MHC-I) antigens can assist in the diagnosis.
MHC-I expression usually occurs early in the disease and continues throughout its course, even with the use of immunosuppression or after clinical remission (8). This underscores that MHC-I and lymphocytic infiltrates are essential markers for the diagnosis and classification of IMs (9).
Lymphocytes have recently been linked to both innate and adaptive immune dysregulation (10). They play a crucial role in adaptive immunity when self-antigens are induced (11-14). The lymphocytic infiltrate in IMs consists of T and B lymphocytes, which are major components in recognizing specific antigens and generating antibody-mediated responses (14). The precise role of T-cells in the pathogenesis of IMs is not yet fully clarified. However, the presence of T-lymphocytes expressing restricted T-cell receptor (TCR) families suggests that these clones can efficiently identify autoantigens in the disease's interaction mechanism (15).
T-cells predominate in the muscle inflammatory infiltrates, with variability in their distribution based on the myositis subset, while B cells are rarely found (12). T-cell subsets include CD4 helper T-cells and CD8 cytotoxic T-cells. CD4+ T-cells recognize major MHC-II presenting peptides, and CD8+ T-cells recognize MHC class I-restricted peptides (12). The predominance of CD4 and CD8 T-cells in IMs is distinct and poses a challenge in determining myositis subtypes. The classical lymphocytic infiltration pattern in DM includes both CD4 T-cells and B-cells prevalent in the perimysium, whereas CD8 T-cells predominantly infiltrate the endomysium in PM with classical MHC-I expression (10).
Because PM is no longer classified as a single spectrum in the updated myositis classification, its diagnosis based on T-cell infiltration is no longer essential (12). A study by Graca and Kouyoumdjian observed that over 75% of DM patients tested positive for CD4 T-cells more frequently than CD8 T-cells (4). CD8 positivity was significant for PM but not for DM (4). The study also concluded that in 15% of DM patients, a diagnosis based solely on inflammatory infiltrate was not possible, aligning with findings reported by Dalakas (8). Furthermore, the study found that more than 50% of DM patients exhibited dense MHC-I expression (4).
Kurdi et al. emphasized in their study that MHC-I expression cannot reliably differentiate IMs from non-IM diseases (16).
Although CD8+ T-cells are predominant in IBM, high levels of T-bet (T-box) and the CD57 marker have been observed in both the muscle tissue and peripheral blood of IBM patients (17, 18). The infiltration of high levels of CD8+ T-cells in IBM predicts a poor response to steroid treatment, which is no longer recommended for most IBM patients (15). Other pro-inflammatory T-cell biomarkers have been investigated in several studies, but their clinical significance and utility remain underappreciated (14).
B-cells are rare in IMs, although local maturation of B-cells and plasma cells may occur in myositis, with B-cells acting as antigen-presenting cells (12). Both B-cells and plasma cells have been reported in PM, DM, and IBM (10, 12, 19). CD4+ T-cells, along with CD19+ B-cells, are commonly observed in DM, suggesting that the BAFF/BAFF-R pathway contributes to both T and B cell responses (19). However, evaluating B-cells in muscle biopsies may not significantly impact the clinical diagnosis.
Some studies have hypothesized that CD8+ T-cells and CD4+ T-cells might be related to viral infections, potentially activated following an autoimmune reaction (20, 21). Therapeutic strategies for IMs often focus on suppressing or modifying immune cell activity, particularly targeting CD4 and CD8 T-cell populations (22).
2. Objectives
This study aims to explore the predominant immune T-cell subpopulations in DM and IBM. Additionally, it seeks to summarize the most frequent clinico-pathological features associated with these two common subsets.
3. Methods
This research study has been ethically approved by the review board committee at King Abdulaziz University, Faculty of Medicine (reference no. 24033). All patients provided consent for the biopsy prior to the procedure. The retrospective cohort included 34 patients, with biopsies and reports obtained from the neuromuscular pathology diagnostic unit at King Fahad Medical Center, King Abdulaziz University, Saudi Arabia, during the period from 2022 to 2023. Only patients diagnosed with IMs were included, consisting of 9 patients with DM, 9 with IBM, and 16 with NSIM (Table 1). The diagnosis was confirmed through muscle biopsy.
| Age | G | Biopsy Site | Muscle Predominance | Symmetry | Rash | Bulbar | CK Level | EMG Finding | Inflammation (%) | Site | T-cell Predominance | Dx |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 47 | F | Biceps | Proximal weakness | Symmetry | Present | Absent | 300 - 999 | Irritative myopathy | 60 | P & E | CD4 | DM |
| 34 | F | Biceps | Proximal & distal weakness | Symmetry | Absent | Absent | 1000 - 2999 | Irritative myopathy | 55 | E | CD8 | DM |
| 47 | M | Quadriceps | Proximal weakness | Symmetry | Present | Absent | 1000 - 2999 | Irritative myopathy | 70 | P & E | CD4 | DM |
| 25 | F | Deltoid | Proximal weakness | Symmetry | Present | Present | 1000 - 2999 | Irritative myopathy | 80 | E | CD4 | DM |
| 43 | F | Biceps | Proximal weakness | Symmetry | Absent | Absent | > 7000 | Irritative myopathy | 40 | P & E | CD4 | DM |
| 40 | M | Biceps | Proximal weakness | Symmetry | Present | Absent | > 7000 | Irritative myopathy | 70 | P & E | CD4 | DM |
| 80 | M | Biceps | Proximal weakness | Symmetry | Absent | Absent | 1000 - 2999 | Irritative myopathy | 60 | P & E | CD8 | DM |
| 19 | F | Quadriceps | Proximal weakness | Symmetry | Present | Absent | 1000 - 2999 | Irritative myopathy | 80 | P | CD4 | DM |
| 28 | M | Biceps | Proximal weakness | Symmetry | Absent | Absent | 3000 - 7000 | Irritative myopathy | 80 | E | CD8 | DM |
| 30 | M | Quadriceps | Proximal weakness | Symmetry | Absent | Absent | 1000 - 2999 | Myopathy | 70 | E & P | CD4 | IBM |
| 68 | F | Biceps | Proximal weakness | Asymmetrical | Absent | Absent | 1000 - 2999 | Irritative myopathy | 70 | E | CD8 | IBM |
| 71 | F | Deltoid | Proximal & distal weakness | Asymmetrical | Absent | Present | 300 - 999 | Irritative Myopathy | 65 | E | CD8 | IBM |
| 47 | M | Biceps | Proximal & distal weakness | Symmetry | Absent | Absent | 300 - 999 | Irritative myopathy | 70 | E | CD8 | IBM |
| 38 | M | Deltoid | Proximal & distal weakness | Symmetry | Absent | Absent | 1000 - 2999 | Myopathy | 30 | E | CD8 | IBM |
| 70 | F | Quadriceps | Proximal weakness | Symmetry | Absent | Absent | 1000 - 2999 | Irritative myopathy | 70 | E | CD8 | IBM |
| 33 | F | Hamstring | Generalized fatigability | Symmetry | Absent | Absent | 1000 - 2999 | Neuromyopathy | 55 | E & P | CD8 | IBM |
| 40 | F | Quadriceps | Generalized fatigability | Symmetry | Absent | Absent | 1000 - 2999 | Nonspecific | 50 | E | CD4 | IBM |
| 55 | M | Quadriceps | Proximal & distal weakness | Symmetry | Absent | Absent | 300 - 999 | Irritative myopathy | 80 | E & P | CD4 | IBM |
| 16 | F | Biceps | Proximal & distal weakness | Symmetry | Absent | Absent | 1000 - 2999 | Irritative myopathy | 30 | E & P | CD4 | NSIM |
| 56 | F | Biceps | Proximal weakness | Symmetry | Absent | Absent | 300 - 999 | Irritative myopathy | 70 | E | CD4 | NSIM |
| 58 | F | Quadriceps | Proximal & distal weakness | Symmetry | Absent | Absent | Normal | Neuromyopathy | 70 | E | CD8 | NSIM |
| 49 | M | Deltoid | Proximal weakness | Symmetry | Absent | Absent | 1000 - 2999 | Irritative myopathy | 10 | P | CD4 | NSIM |
| 60 | F | Quadriceps | Proximal weakness | Symmetry | Absent | Absent | 1000 - 2999 | Neuromyopathy | 40 | E | CD8 | NSIM |
| 32 | F | Hamstring | Generalized fatigability | Symmetry | Absent | Absent | 1000 - 2999 | Neuromyopathy | 55 | E & P | CD8 | NSIM |
| 44 | M | Quadriceps | Generalized fatigability | Symmetry | Absent | Absent | 1000 - 2999 | Nonspecific | 50 | E | CD4 | NSIM |
| 50 | F | Biceps | Proximal weakness | Symmetry | Present | Absent | 300 - 999 | Myopathy | 20 | P | CD4 | NSIM |
| 32 | M | Biceps | Proximal weakness | Symmetry | Absent | Absent | > 7000 | Irritative myopathy | 80 | E | CD8 | NSIM |
| 33 | M | Biceps | Proximal weakness | Symmetry | Absent | Absent | > 7000 | Irritative myopathy | 80 | E | CD8 | NSIM |
| 38 | F | Quadriceps | Proximal & distal weakness | Symmetry | Absent | Absent | 3000 - 7000 | Irritative myopathy | 90 | E & P | CD4 | NSIM |
| 50 | M | Quadriceps | Proximal weakness | Symmetry | Absent | Absent | 300 - 999 | Myopathy | 20 | P | CD4 | NSIM |
| 70 | M | Quadriceps | Proximal weakness | Symmetry | Absent | Absent | 3000 - 7000 | Irritative myopathy | 30 | P | CD4 | NSIM |
| 19 | F | Quadriceps | Proximal weakness | Symmetry | Present | Absent | 300 - 999 | Irritative myopathy | 80 | E | CD4 | NSIM |
| 33 | F | Quadriceps | Proximal weakness | Symmetry | Absent | Absent | > 7000 | Irritative myopathy | 20 | E | CD8 | NSIM |
| 61 | M | Quadriceps | Proximal weakness | Symmetry | Absent | Absent | 3000 - 7000 | Myopathy | 30 | P | CD4 | NSIM |
Descriptive Data of All Patients Enrolled in This Study (Last Page)
The study included adult patients of both genders with muscle biopsy sizes greater than 1.0 cm in dimension. Their primary clinical presentation was muscle weakness. Muscle biopsy tissue processing was performed using the frozen tissue technique with liquid nitrogen, followed by sectioning through a cryostat machine. The essential histological features in all cases included myonecrosis, regeneration, and inflammation. Histochemical staining and immunohistochemistry (IHC) were also performed. All cases were retrospectively examined for CD4, CD8, and MHC-I using IHC. The presence of mitochondrial abnormalities, such as ragged-red fibers, blue-ragged fibers, and cytochrome oxidase (COX)-negative fibers, was also evaluated. All findings and results were obtained from muscle biopsy reports and reviewed by a certified neuropathologist (MK). The summarized results are presented in (Table 1).
The relationships between immune T-cell subpopulations, histological features, and IM subsets were analyzed using the Pearson chi-square test and Fisher exact test with IBM SPSS version 24 (SPSS Inc., Chicago, IL, USA). A P-value of less than 0.05 was considered statistically significant.
4. Results
The mean age of the patients in this study was 44 years (SD: 19.82), with 17 males and 17 females. The cases were categorized into three groups: Dermatomyositis (n = 9), IBM (n = 9), and NSIM (n = 16). Nonspecific IMs cases displayed no distinct features characteristic of DM, IBM, or other immune-mediated myopathies, and MSAs were not included in this investigation to avoid conflicts in statistical analysis. The duration of the patients’ symptoms ranged from 4 weeks and two years.
Approximately 94% of patients presented with symmetrical muscle weakness, with 61.1% exhibiting a proximal distribution. Rash was observed in seven patients (5 DM cases and 2 NSIM cases). Bulbar symptoms were identified in two patients (one DM and one IBM). Creatine kinase (CK) levels exceeded 3000 mu/mL in nine patients, and an irritative myopathic pattern on electromyography (EMG) was diagnosed in 23 patients.
Muscle biopsies were predominantly taken from upper limb muscles (biceps and deltoid) in 50% of cases, with the remainder taken from lower limb muscles (mostly quadriceps). All muscle biopsies demonstrated necrotic and regenerating muscle fibers, as well as chronic inflammatory infiltrates of varying degrees. Inflammatory infiltrates in DM patients were primarily located in the perimysium and endomysium, whereas in IBM patients, inflammation was predominantly observed in the endomysial spaces (Table 1).
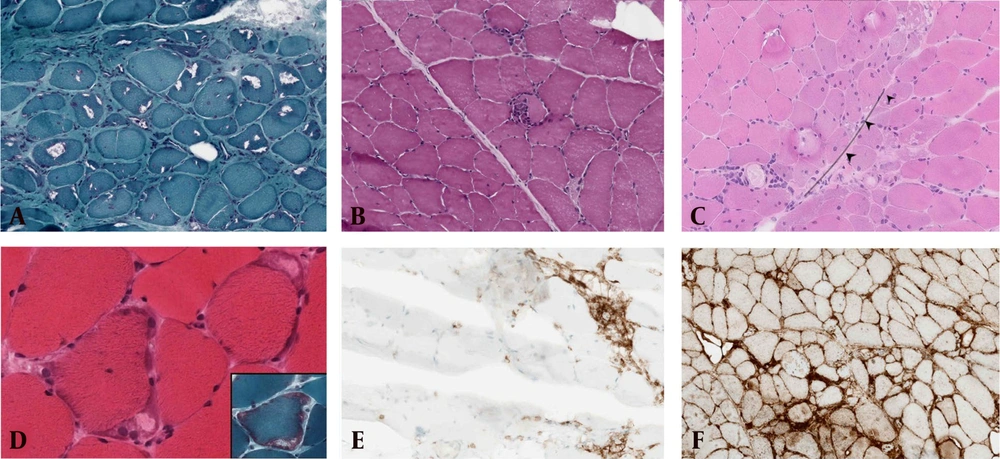
Distinct and predominant histological features associated with IBM and DM are illustrated in Figure 1.
Common histological features associated with dermatomyositis (DM) and inclusion body myositis (IBM). A, rimmed vacuoles seen in IBM; B, lymphocytic invasion to muscle fiber in IBM; C, perifascicular pathology (PFP) seen in DM – this image was used with permission from DR. Maher Kurdi, Neuromuscular pathology book, Taylor and Francine, First edition, 2020 - 21; D, subsarcolemmal mitochondrial aggregation seen in IBM; E, CD4 predominant perivascular inflammation seen in DM; F, widespread expression of MHC-Class I expression in fibers with IBM.
There was no statistically significant difference in the distribution of CD4 and CD8 T-cells among DM, IBM, and NSIM patients (P-value = 0.358) (Table 2). However, CD4+ T-cells were observed more frequently in DM patients (6 / 9) compared to IBM, whereas CD8+ T-cells predominated in IBM patients (6 / 9) compared to DM (Table 3). Nonspecific inflammatory myopathies patients exhibited a higher presence of CD4+ T-cells than CD8+ T-cells.
| Variables | Predominant Inflammation | Total | Cum P-Value | |
|---|---|---|---|---|
| CD4 | CD8 | |||
| Histopathological Diagnosis | 0.358 | |||
| DM | 6 (37.5) | 3 (20.0) | 9 (29.0) | |
| IBM | 3 (18.8) | 6 (40.0) | 9 (29.0) | |
| NSIM | 10 (43.8) | 6 (40.0) | 16 (41.9) | |
Predominant Histopathological Characteristic Features of Inflammatory Myopathy Subsets
| Variables | DM | IBM | NSIM |
|---|---|---|---|
| Predominant CD4 expression | 6 | 3 | 10 |
| Predominant CD8 expression | 3 | 6 | 6 |
| Protein aggregates | 0 | 5 | 3 |
| Rimmed vacuoles | 0 | 5 | 0 |
| MHC-I expression | 6 (perifascicular) | 9 (widespread) | 14 (widespread) |
| Mitochondrial abnormalities | 1 | 6 | 1 |
| Perifascicular pathology | 9 | 0 | 1 |
| Neuropathic change | 0 | 5 | 5 |
Predominant Histopathological Characteristic features of Inflammatory Myopathy Subsets
A significant statistical difference was found in the patterns of MHC-I expression and mitochondrial abnormalities between DM, IBM, and NSIM (P-value < 0.05) (Tables 4 and 5). Perifascicular pathology was present in all DM patients, while none of the IBM cases exhibited PFP. Conversely, 5 / 9 IBM patients displayed rimmed vacuoles in their muscle biopsies, while no DM cases exhibited rimmed vacuoles or eosinophilic protein aggregates. In NSIM, protein aggregates were observed in three patients, though their clinical significance remains uncertain.
| Variables | MHC-I Expression Pattern | Total | Cum P-Value | ||
|---|---|---|---|---|---|
| Unremarkable | Per Fascicular | Widespread | |||
| Histopathological Diagnosis | < 0.001 | ||||
| DM | 0 (0.0) | 9 (100.0) | 0 (0.0) | 9 (26.5) | |
| IBM | 0 (0.0) | 0 (0.0) | 9 (39.1) | 9 (26.5) | |
| NSIM | 2 (100.0) | 0 (0.0) | 14 (60.9) | 16 (47.1) | |
MHC-I Expression Pattern Among all Inflammatory Myopathy Subsets
| Variables | Mitochondrial Abnormalities | Total | Cum P-Value | |
|---|---|---|---|---|
| Absent | Present | |||
| Histopathological Diagnosis | 0.009 | |||
| DM | 8 (32.0) | 1 (11.1) | 9 (26.5) | |
| IBM | 3 (12.0) | 6 (66.7) | 9 (26.5) | |
| NSIM | 14 (56.0) | 2 (22.2) | 16 (47.1) | |
Mitochondrial Abnormalities in All Inflammatory Myopathy Subsets
Mitochondrial abnormalities were identified in 6 / 9 IBM patients. In DM, mitochondrial changes were observed in a single case, likely attributable to perifascicular regeneration or necrosis. Neuropathic changes were detected in 5 IBM patients and 5 NSIM patients, whereas no neuropathic components were observed in DM cases (Table 3).
5. Discussion
Numerous studies have extensively discussed the characteristic features of DM and IBM. However, the specific immune T-cell subpopulations involved in these subsets of myositis remain poorly understood due to limited case reports and research studies. Immune T-cell subpopulations play a crucial role in recognizing specific antigens and generating antibody-mediated responses in IMs, though their exact contribution to the pathogenesis of these diseases is still unclear.
B-cell infiltration in IMs is rare and considered clinically insignificant. T-cell subpopulations consist of CD4+ T-cells and CD8+ T-cells, with CD4+ T-cells recognizing MHC-II presenting receptors and CD8+ T-cells recognizing MHC class I-restricted peptides. While CD4 and CD8 are traditionally used to differentiate between DM and IBM, the composition of the inflammatory infiltrate can vary among patients.
A recent study by Graca and Kouyoumdjian observed that patients with DM commonly tested positive for CD4+ T-cells, whereas CD8+ T-cells were significantly expressed in PM (4). Similarly, Dimitri et al. identified a significant prevalence of CD8+ T-cells in patients with IBMs (18). Although both CD4 and CD8 T-cells bind to presenting peptides in MHC-I, this interaction is not always diagnostic for DM or IBM. While MHC-I cannot reliably differentiate IMs from non-IMs, its dense expression underscores the autoimmune inflammatory mechanisms involved in these diseases (16).
The presence of a perifascicular pattern of MHC-I is a characteristic histopathological sign in DM (23). The exact mechanisms underlying the perifascicular pattern of MHC-I expression in DM are not fully understood; however, it is believed to be associated with immune-mediated destruction of the blood vessels supplying the muscle fibers (16, 24). Similarly, dense widespread expression of MHC-I is a common feature in IBMs (16).
In our study, we observed that CD4+ T-cells were the predominant immune T-cell subpopulations in DM, while CD8+ T-cells were the main predominant subpopulations in IBM (Table 3). Among NSIMs, no significant immune cell subpopulation was identified. Furthermore, PFP was exclusively identified in DM patients, while mitochondrial abnormalities were a common finding in IBM (Table 3). The exact cause of these abnormalities remains under investigation.
Previous studies have suggested that muscle fibers in IBM may harbor mtDNA mutations, which can impair mitochondrial function and result in deficits in energy production (25). In our study, mitochondrial changes were observed in the majority of IBM patients, while only a single case of DM and NSIM showed rare mitochondrial changes. We hypothesize that the PFP observed in these DM cases may be linked to abnormal mitochondrial damage caused by muscle degeneration and injury. Other predominant pathological features frequently observed in IBM included protein aggregates, rimmed vacuoles, and neuropathic changes (Table 3).
We acknowledge the limitations of our study, particularly the small sample size and the potential underdiagnosis of other NSIMs due to limited access to serological testing for patients. While the histological features identified in this study highlight common characteristics of DM and IBM, the absence of P52 and MAC immunolabeling results may limit the accuracy of the diagnoses.
5.1. Conclusions
Immune T-cell subpopulations and pathological changes between DM and IBM exhibit distinct specific characteristics. CD4+T-cells and PFP are frequently observed in DM, while CD8+T-cells, rimmed vacuoles, and mitochondrial abnormalities are common features in IBM. MHC-I expression varies across all IMs, with a perifascicular pattern predominantly restricted to DM. NSIMs lack specific features; however, performing MSAs is essential for their classification.