1. Background
Parkinson's disease (PD) is the second most common neurodegenerative disorder after Alzheimer's disease (AD), characterized by both motor and non-motor symptoms, including bradykinesia, tremors, rigidity, and cognitive impairments, which collectively impact patients' quality of life (1). A key pathological hallmark of PD is the progressive loss of dopaminergic neurons in the substantia nigra pars compacta (SNC), accompanied by the presence of intraneuronal inclusions known as Lewy bodies (2). In addition to genetic predispositions (3), mitochondrial dysfunction, heightened oxidative stress, and depleted glutathione levels have been identified as major contributors to dopaminergic degeneration in PD (4). Gender differences in PD, potentially linked to estrogen’s influence, have sparked a great deal of curiosity among researchers. Estrogen has demonstrated neuroprotective effects, including protection against amyloid-beta toxicity (5), oxidative injury (6), and mitochondrial dysfunction (7). Phytoestrogens — plant-derived compounds with structural and functional similarities to 17-β estradiol — are a promising area of exploration for their potential antioxidant (8) and neuroprotective properties (9). Among the various families of phytoestrogens, isoflavones, predominantly found in soybeans and legumes, have been extensively studied for their health benefits across multiple systems, including reproductive, cardiovascular, and neurological health (9). Genistein, a prominent isoflavone, has shown potential for modulating cellular pathways involved in inflammation, oxidative stress, and apoptosis (10). Previous research has demonstrated its ability to inhibit intracellular signaling pathways associated with NF-kappa B, suppress microglial activation, and reduce apoptosis in neurodegenerative disease models (11, 12). However, the therapeutic efficacy of genistein, particularly its dose-dependent effects, requires further investigation. In earlier studies, a single low dose of genistein (10 mg/kg) exhibited neuroprotective effects in a 6-OHDA rat model of Parkinsonism.
2. Objectives
Building on this, the current study aims to examine the impact of two additional doses of genistein (50 mg/kg and 500 mg/kg) on motor function, oxidative stress, and neuroinflammation in a similar model. This investigation seeks to elucidate the dose-dependent effects of genistein and its potential as a therapeutic agent in PD.
3. Methods
3.1. Animal Model
This experimental study was approved by the Animal Ethics Committee of Ilam University of Medical Sciences (Approval ID: IR.MEDILAMREC.1395.114). All procedures were conducted under ketamine and xylazine anesthesia, adhering to ethical guidelines to minimize suffering. Male Wistar rats (weighing 200 - 280 g; n=32) were procured from Ilam University, Iran. The animals were housed in temperature-controlled environments with a regulated dark/light cycle and were provided with ad libitum access to food and water. Three rats were housed per transparent plastic cage. Prior to the experiments, rotational behavior was assessed to exclude animals with rotations exceeding 30 per hour. The rats were then randomly assigned to one of four groups (n = 8 per group): Group A (Sham): Rats received normal saline through stereotaxic surgery. Group B (Lesion): Rats were injected with 6-hydroxydopamine (6-OHDA) stereotaxically. Group C (Lesion + Gen50): Rats were pretreated with 50 mg/kg of genistein via oral gavage, followed by 6-OHDA injection. Group D (Lesion + Gen500): Rats were pretreated with 500 mg/kg of genistein via oral gavage, followed by 6-OHDA injection. Genistein was dissolved in Cremophor-EL and administered via oral gavage approximately one hour before surgery. All animals remained healthy throughout the study, with no unplanned mortality observed. After the behavioral, biochemical, and histochemical assessments, the animals were sacrificed, and their brains were stored at -70°C for further analysis. All evaluations were conducted by researchers blinded to the treatment groups.
3.2. Surgical Procedures
Anesthesia was induced with a combination of ketamine (100 mg/kg) and xylazine (5 mg/kg). Each rat was secured in a stereotaxic apparatus, and the skull was exposed. Using a Hamilton syringe, 5 µL of 0.9% saline containing 2.5 µg/µL of 6-OHDA-HCl and 0.2% ascorbic acid (w/v), was slowly injected unilaterally at coordinates based on the Paxinos and Watson atlas (L: -3 mm, AP: +9.2 mm, V: +4.5 mm from the interaural line). Post-surgery, animals were monitored in a clean, dry environment to ensure recovery, with ventilation and thermoregulation closely observed. They were transferred to the animal facility once fully recovered. Analgesics were not administered post-surgery. All procedures were performed between 8:00 AM and 12:00 PM.
3.3. Behavioral Testing
Two weeks post-surgery, rotational behavior was assessed in all groups. Apomorphine hydrochloride (2 mg/kg) was administered intraperitoneally, and the animals were placed in cylindrical Plexiglas containers. Rotational behavior was recorded for one hour at 10-minute intervals. Net rotational counts were calculated by subtracting counterclockwise rotations from clockwise rotations. Apomorphine-induced rotational testing evaluates functional deficits associated with dopaminergic asymmetry and degeneration in the SNc. Notably, neurodegeneration was evaluated using histological, behavioral, and biochemical assessments.
3.4. Biochemical Study
The rats were deeply anesthetized with ketamine (150 mg/kg) before decapitation. Brains were immediately removed, and the basal ganglia were homogenized (5% tissue homogenates in 0.9% saline), centrifuged at 1000 × g for 10 minutes at 4°C, and stored at -80°C for subsequent biochemical analysis.
3.5. Measurement of Superoxide Dismutase Activity
Superoxide dismutase (SOD) activity was assessed using the Nitroblue tetrazolium (NBT) reduction assay, a widely used method for measuring the activity of this important antioxidative enzyme as previously described (13). In this assay, brain homogenate supernatants were prepared from tissue samples and incubated with xanthine and xanthine oxidase in potassium phosphate buffer (PBS, pH 7.8) at 37°C for 40 minutes. This incubation step facilitates the generation of superoxide radicals, which are the substrates for SOD. Following incubation, NBT was added to the reaction mixture. The NBT reduction is indicative of superoxide radical scavenging, and the development of a blue color is directly related to the extent of the reaction. Spectrophotometric measurements of the color development were taken at 550 nm, which corresponds to the absorbance peak of the reduced NBT for precise quantification. Superoxide dismutase activity was calculated based on the protein concentration required to inhibit NBT reduction by 50%, which is referred to as one nitrite unit of SOD activity. This value represents the amount of SOD that reduces superoxide radicals in the reaction, thereby preventing the NBT reduction. This method provides a reliable means of quantifying SOD activity and offers a quantitative measure of antioxidative capacity in the brain homogenates.
3.6. Lipid Peroxidation Assay (Malondialdehyde Measurement)
Lipid peroxidation was evaluated by quantifying malondialdehyde (MDA) levels using a commercial Rat MDA ELISA Kit (A247177), following the manufacturer’s instructions. This method involves the formation of a yellow-colored product proportional to the MDA concentration upon addition of a stop solution. The absorbance was measured at 450 nm to determine MDA levels in the brain homogenates.
3.7. DNA Fragmentation Assay (Apoptosis)
Apoptosis was assessed by measuring histone-associated DNA fragments using the Cell Death Detection ELISA kit (Roche Diagnostics, Germany). The procedure adhered to the manufacturer’s protocol and a previously established method (Ghofrani, 2015). This assay utilized monoclonal antibodies specific to DNA and histones. The retained peroxidase in the immunocomplex was quantified photometrically at 405 nm using a microplate reader (BioTek, USA).
3.8. TNF-α Quantification
Tumor necrosis factor-alpha (TNF-α) levels were measured using a Rat TNF-α ELISA Kit (E0764Ra) according to the manufacturer’s protocol. The assay sensitivity was 2.51 ng/L, with a detection range of 5 - 1000 ng/L. The microplate, pre-coated with Rat TNF-α antibodies, captured the cytokine in the samples. Biotinylated antibodies and Streptavidin-HRP were used to enhance detection. After substrate addition, absorbance was recorded at 450 nm to determine TNF-α concentrations.
3.9. Histochemical Analysis
3.9.1. Transcranial Perfusion and Sectioning
Animals were deeply anesthetized and transcardially perfused with 4% paraformaldehyde in PBS. Brains were subsequently embedded in paraffin after fixation. Three days later, 20-μm coronal brain sections were prepared using a microtome and placed on glass slides. Nissl staining was performed to visualize neuronal structures.
3.9.2. Neuronal Counting
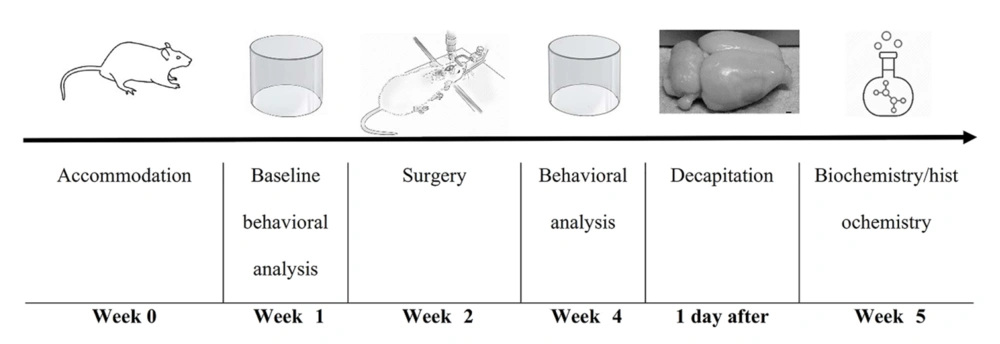
Neuron counts in the SNc were performed on Nissl-stained brain sections. Coronal sections were collected from regions spanning from -3.3 mm to -4.5 mm relative to the bregma, following the Paxinos and Watson atlas for anatomical guidance. This region of the brain is critical for evaluating dopaminergic neuronal loss, which is a hallmark of Parkinson’s disease. For each animal, 3 to 4 coronal brain sections were selected for analysis. To ensure accuracy, three fields per section were examined at a magnification of 40× using light microscopy. Only intact, Nissl-positive neurons were counted, as these cells are indicative of viable neurons with preserved cell bodies. Nissl staining highlights the rough endoplasmic reticulum and provides a reliable marker for intact neurons. Neuron counting was performed using ImageJ software, which allows for accurate and reproducible measurements. To minimize bias, the evaluator was blinded to the treatment groups, ensuring that the analysis remained objective and independent of group allocation. This methodological approach enabled a detailed and systematic assessment of neuronal survival in the SNc, a region that is particularly vulnerable to neurodegeneration in Parkinson’s disease models. The study timeline is illustrated in Figure 1. In addition, the flow diagram of the study design is illustrated in Table 1.
| Step | Description | Details |
|---|---|---|
| 1. Ethics approval | Approval from animal ethics committee | Approval ID: IR.MEDILAMREC.1395.114 |
| 2. Animal selection | Male Wistar rats (200 - 280 g; n = 32) | Housed in temperature-controlled environment, ad libitum access to food and water. |
| 3. Behavioral screening | Pre-screening for rotation behavior | Exclude rats with >30 rotations/hour |
| 4. Randomization into 4 groups | Random allocation to one of four groups (n = 8/group) | Group A (Sham): Saline + surgery; group B (Lesion): 6-OHDA + no treatment; group C (Lesion + Gen50): 50 mg/kg genistein + 6-OHDA; group D (Lesion + Gen500): 500 mg/kg genistein + 6-OHDA |
| 5. Surgical procedure | 6-OHDA lesion via stereotaxic surgery | Anesthesia: Ketamine (100 mg/kg), Xylazine (5 mg/kg). 6-OHDA injected into the substantia nigra (5 µL). |
| 6. Post-surgery recovery | Monitoring and recovery | No unplanned mortality observed. |
| 7. Behavioral analysis | Two weeks post-surgery | Apomorphine (2 mg/kg) administered. Rotational behavior measured for 1 hour at 10-min intervals. |
| 8. Biochemical analysis | Measurement of oxidative stress and neuroinflammation | SOD Activity: NBT reduction assay ; MDA Levels: ELISA ; DNA Fragmentation: ELISA; TNF-α Levels: ELISA |
| 9. Histological analysis | Nissl staining for neuronal structures | Coronal brain sections stained and counted for neuron survival in substantia nigra compacta (SNc). |
| 10. Data analysis | Statistical analysis | One-way ANOVA or Kruskal-Wallis test for behavioral data. Statistical significance set at P < 0.05. |
a The diagram illustrates the experimental workflow, including animal selection, group allocation, surgical procedures, behavioral assessments, biochemical and histological analyses. Male Wistar rats (n = 32) were randomly assigned to four groups: Sham (saline control), Lesion (6-OHDA injection), Lesion + Gen50 (50 mg/kg genistein + 6-OHDA), and Lesion + Gen500 (500 mg/kg genistein + 6-OHDA). Following treatment and behavioral assessments, animals were sacrificed for biochemical and histological evaluations.
3.10. Statistical Analysis
All data were expressed as mean ± SEM. Biochemical parameters were analyzed using one-way analysis of variance (ANOVA) followed by Tukey’s post-hoc test for group comparisons. Non-parametric data from behavioral studies were analyzed with the Kruskal-Wallis test, followed by Dunn’s post-hoc test where significant differences were identified. Statistical significance was set at P < 0.05. All analyses were performed using GraphPad Prism 8.
4. Results
4.1. Genistein Treatment Improves Rotational Behavior in 6-OHDA-lesioned Rats
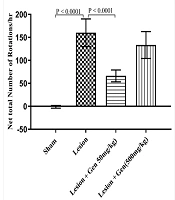
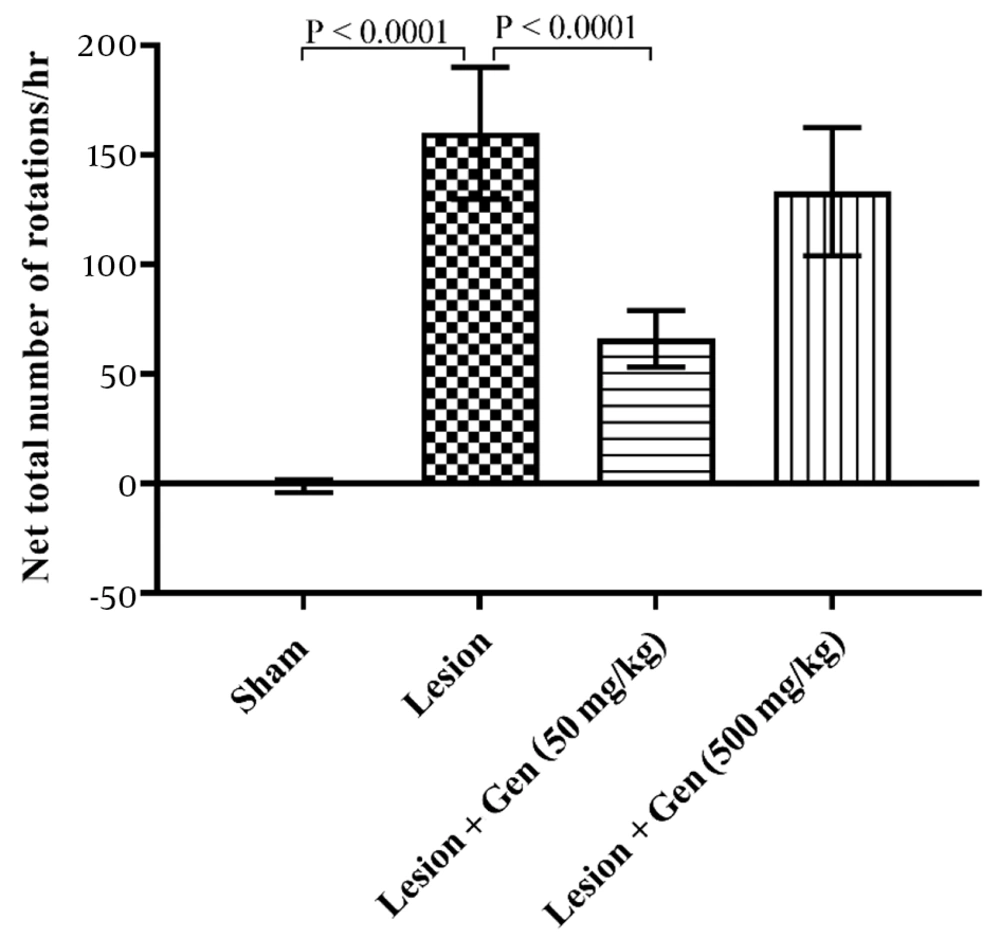
Rats injected with 6-OHDA exhibited a significant increase in contralateral rotations (160 ± 30.1) compared to the sham-operated group (-1.2 ± 2.9; P < 0.0001). Pretreatment with 50 mg/kg genistein significantly reduced rotational behavior (66.1 ± 12.9; P < 0.0001), while 500 mg/kg genistein showed no significant difference from the lesioned group (133.3 ± 29.2; Figure 2).
Genistein treatment improves rotational behavior in 6-OHDA-lesioned rats. The group that received a 6-OHDA injection exhibited a significant increase in rotational behavior compared to the sham-operated group (P < 0.0001). Animals pretreated with 50 mg/kg of genistein showed a significant decrease in contralateral rotations vs. the lesioned animals (P < 0.0001). However, rats pretreated with 500 mg/kg of genistein did not show a significant reduction in rotational behavior compared to the lesioned group.
4.2. Genistein Modulates Oxidative Stress by Altering Superoxide Dismutase Activity in Parkinson's Disease Model
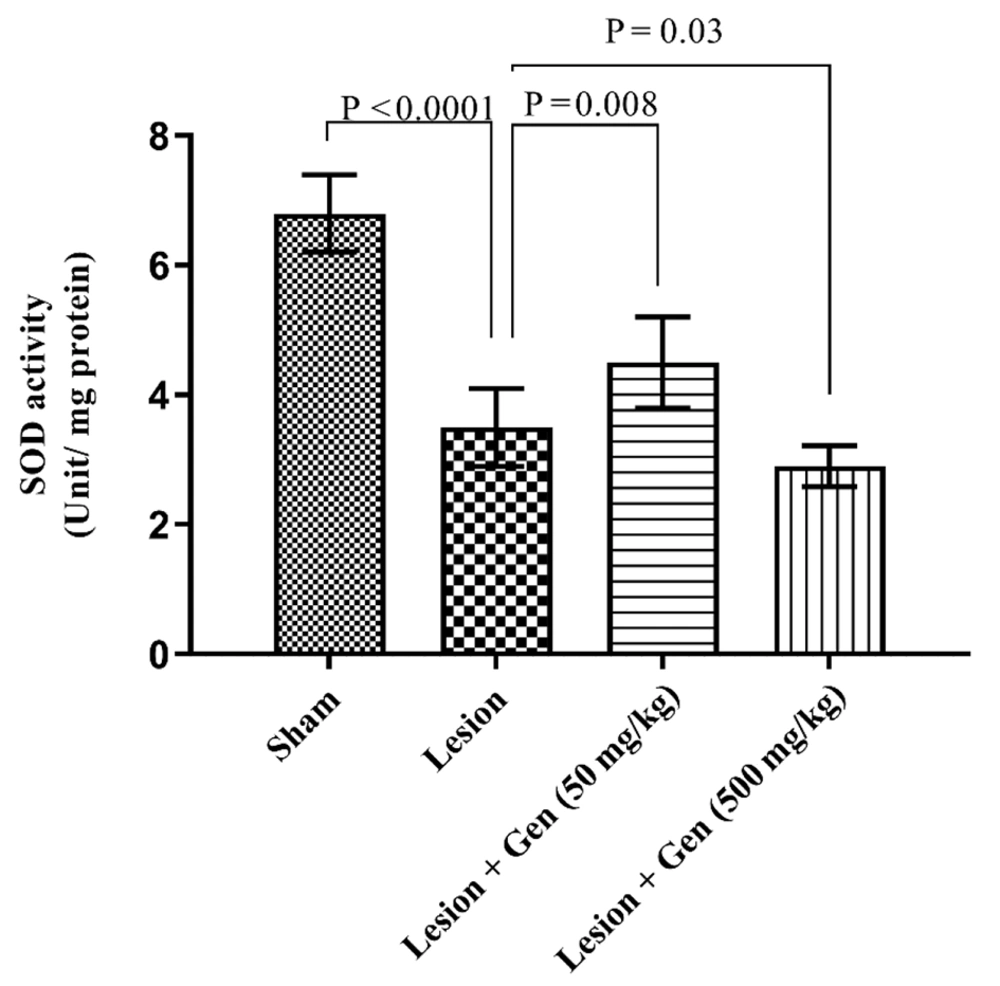
The SOD activity varied significantly across experimental groups. In the sham-operated animals, SOD activity was measured at 6.8 ± 0.6 units/mg protein. In contrast, rats injected with 6-OHDA exhibited a marked reduction in SOD activity, recording 3.5 ± 0.6 units/mg protein, a significant decrease compared to the sham group (P < 0.0001). Pretreatment with 50 mg/kg of genistein partially restored SOD activity to 4.5 ± 0.7 units/mg protein, representing a significant improvement over the lesioned group (P = 0.008). Conversely, administration of 500 mg/kg of genistein further reduced SOD activity to 2.9 ± 0.32 units/mg protein, significantly lower than the lesioned group without pretreatment (P = 0.03). These findings, depicted in Figure 3, suggest a dose-dependent effect of genistein on SOD activity in 6-OHDA-injected animals.
Genistein modulates oxidative stress by altering Superoxide dismutase (SOD) activity in Parkinson's disease (PD) model. SOD activity (Units/mg protein) was significantly higher in animals with 6-OHDA injection compared to the sham group (P < 0.0001). Genistein pretreatment at 50 mg/kg further increased SOD activity significantly compared to lesioned animals (P = 0.008). However, pretreatment with 500 mg/kg of genistein resulted in a significant decrease in SOD activity compared to lesioned animals (P = 0.03).
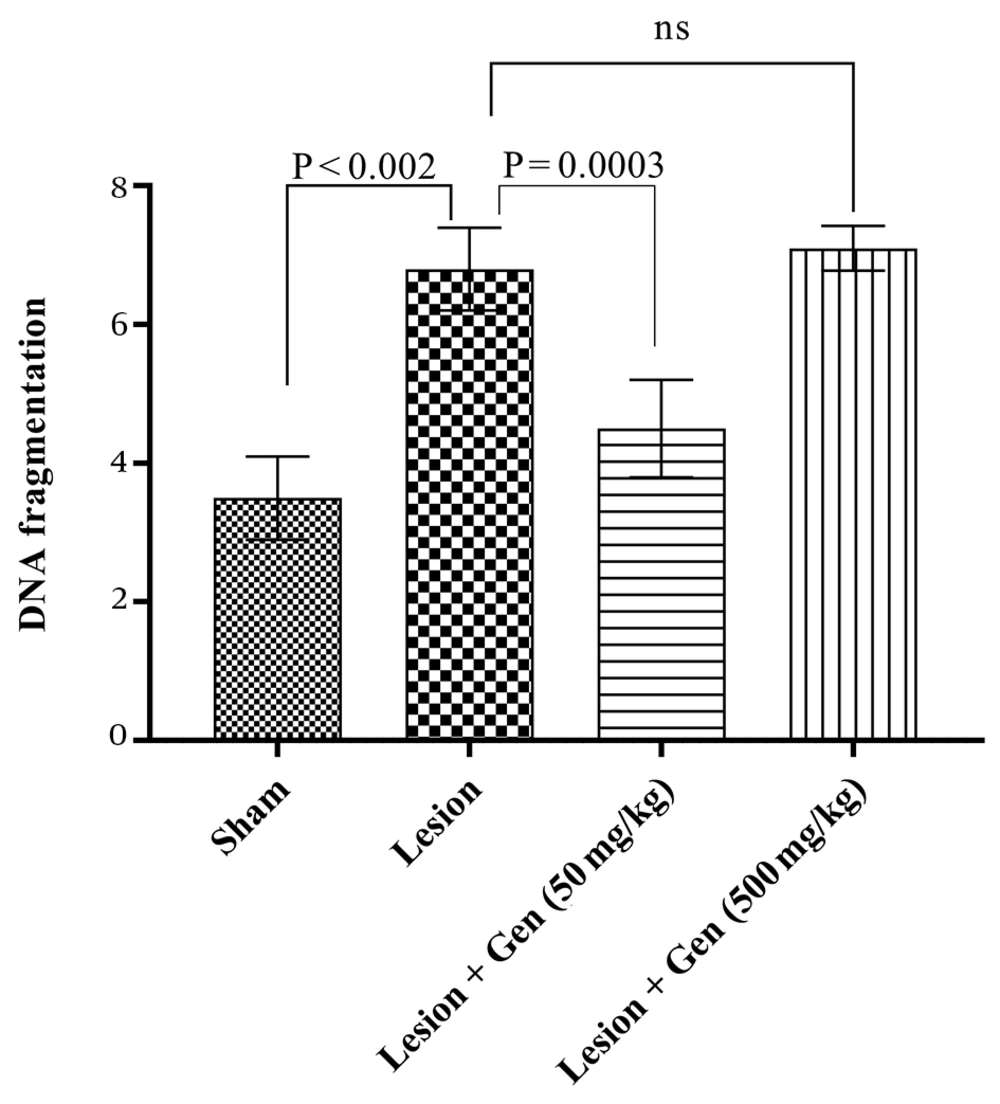
4.3. Effects of Genistein on DNA Fragmentation and Apoptosis in Parkinson's Disease Rats
Chromosomal DNA fragmentation, a well-established marker of apoptosis, was significantly increased in the 6-OHDA-injected group compared to the sham group (6.8 ± 0.6; P < 0.002). Pretreatment with genistein at a dose of 50 mg/kg led to a significant reduction in DNA fragmentation compared to the lesioned group (4.5 ± 0.7; P = 0.0003). Interestingly, a higher dose of genistein (500 mg/kg) exacerbated DNA fragmentation, with levels reaching 7.1 ± 0.3, indicating a potential dose-dependent adverse effect. These results are illustrated in Figure 4.
Effects of genistein on DNA fragmentation and apoptosis in Parkinson's disease (PD) Rats. Measurement of chromosomal DNA breakdown showed a significant increase in DNA fragmentation in the 6-OHDA-injected group compared to the sham group (P < 0.002). Pretreatment with 50 mg/kg genistein reduced DNA fragmentation, though not significantly. However, pretreatment with 500 mg/kg genistein significantly elevated DNA fragmentation levels compared to the lesioned group (P = 0.0003).
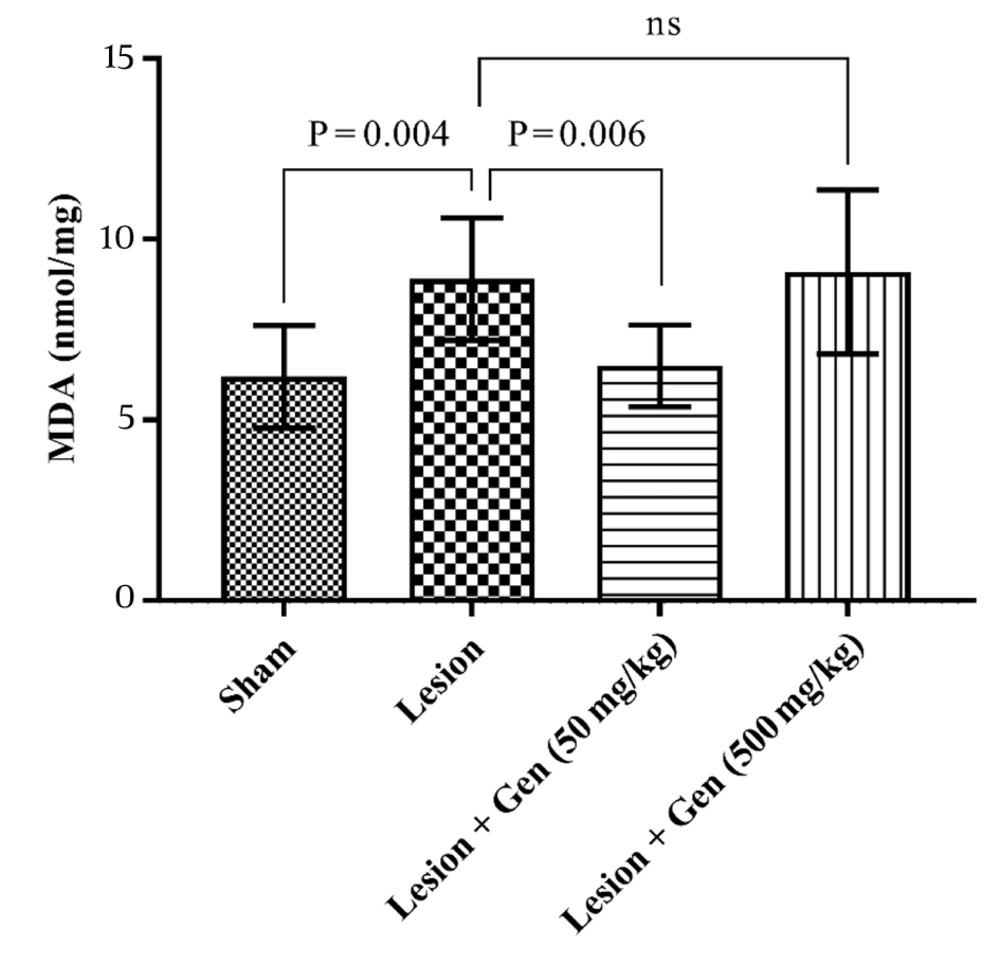
4.4. Genistein Influences Lipid Peroxidation by Modifying Malondialdehyde Levels
The MDA, a marker for lipid peroxidation, was also evaluated across all groups. In the sham group, MDA levels were recorded at 6.2 ± 0.5 nmol/mg, while in the lesioned group, these levels significantly increased to 8.9 ± 0.6 nmol/mg (P = 0.004). Pretreatment with 50 mg/kg of genistein reduced MDA levels to 6.5 ± 0.4 nmol/mg, a significant decrease compared to the lesioned group (P = 0.006). However, administration of 500 mg/kg of genistein elevated MDA levels to 9.1 ± 0.8 nmol/mg, showing no significant difference from the lesioned group. These results, illustrated in Figure 5, underscore the dose-dependent variability of genistein's effects on lipid peroxidation.
Genistein influences lipid peroxidation by modifying MDA levels in Parkinson's disease (PD) model. Malondialdehyde (MDA) levels, a marker of lipid peroxidation, were significantly increased in lesioned animals compared to sham-operated rats (P = 0.004). In the genistein pretreatment group at 50 mg/kg, MDA levels showed a significant decrease compared to the lesioned group (P = 0.006). However, pretreatment with 500 mg/kg of genistein did not change MDA levels compared to the lesioned group.
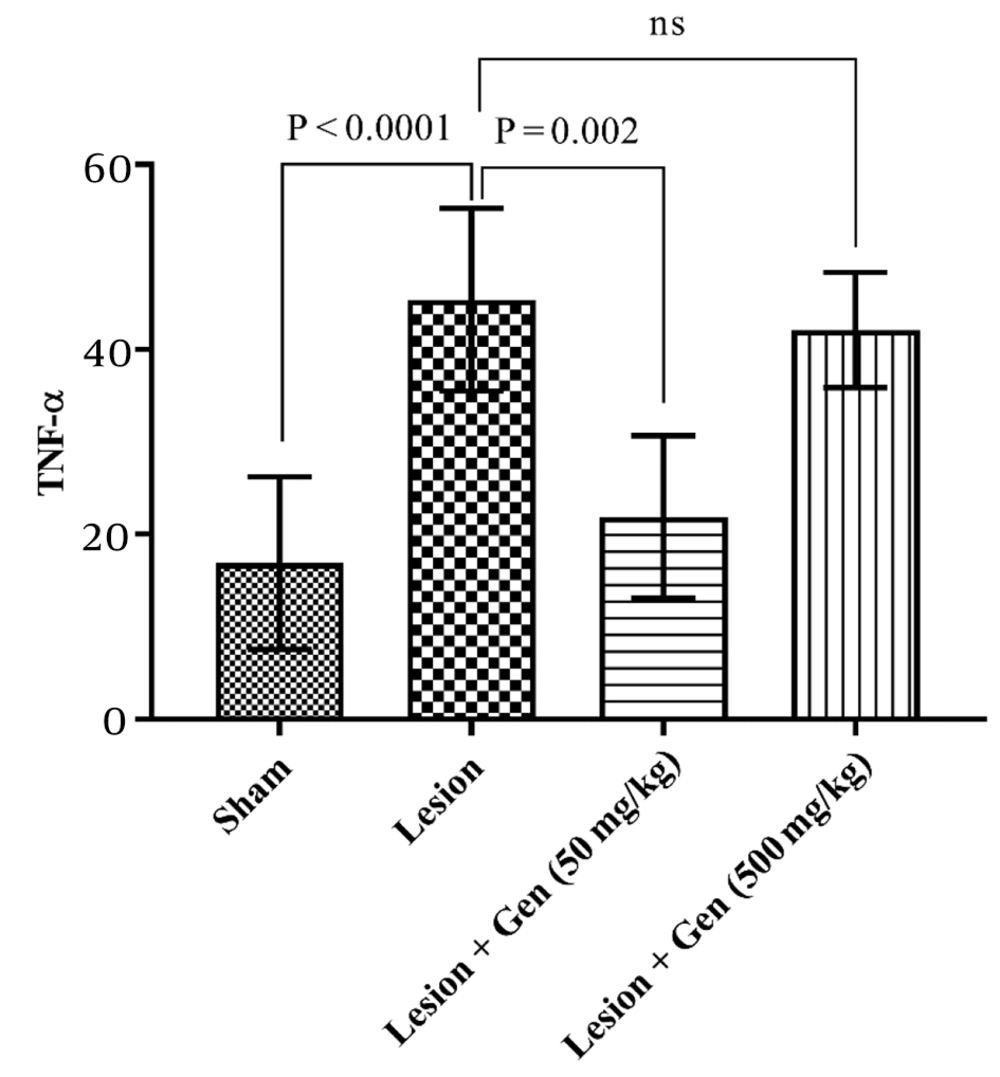
4.5. Impact of Genistein on TNF-α Levels and Neuroinflammation in Parkinson's Disease Model
Tumor necrosis factor-alpha (TNF-α) levels were also assessed to evaluate inflammation across the groups. TNF-α content in the sham-operated group was 16.9 ± 6.3. This value increased significantly in the lesioned animals to 45.4 ± 3.5 (P < 0.0001). Pretreatment with 50 mg/kg genistein significantly decreased TNF-α levels to 21.9 ± 3.1 (P = 0.002). However, in animals pretreated with 500 mg/kg genistein, TNF-α levels remained elevated at 42.1 ± 2.2, showing no significant difference compared to the lesioned group. These results are depicted in Figure 6, highlighting the differential impact of genistein dosing on inflammation markers.
Impact of genistein on TNF-α levels and neuroinflammation in 6-OHDA-lesioned rats. TNF-α levels were significantly increased in lesioned animals compared to sham-operated rats (P < 0.0001). Pretreatment with 50 mg/kg genistein significantly decreased TNF-α levels compared to the lesioned group (P = 0.002). However, pretreatment with 500 mg/kg genistein did not result in a significant change in TNF-α levels compared to the lesioned group.
4.6. Neuroprotective Effects of Genistein on Nissl-stained Neurons in Substantia Nigra Pars Compacta
The neuronal count in the SNc revealed a significant loss in the 6-OHDA-lesioned rats, with an average of 70.6 ± 9.8 neurons, compared to 125 ± 13.6 neurons in the sham-operated controls (P = 0.001). This highlights the detrimental effect of the 6-OHDA lesion on dopaminergic neurons in this brain region. Interestingly, pretreatment with 50 mg/kg of genistein resulted in a noticeable improvement, with the neuron count increasing to 109 ± 8.6, significantly higher than the lesioned group (P = 0.01). However, pretreatment with a higher dose of genistein (500 mg/kg) did not provide any additional benefit, as the neuron count remained similar to that of the lesioned group, at 72.7 ± 8.1. These findings suggest that a lower dose of genistein may have a protective effect against neuronal loss, while the higher dose might not offer any further neuroprotection. These results are summarized in Table 2 and offer insight into the potential dose-dependent effects of genistein on neuroprotection.
a The mean number of Nissl-stained neurons 2 weeks after the experiment in the left and right sides of brain. There was not any significant difference between the mean number of neurons in the rt side of substantia nigra in all groups
b Significant difference in lesioned rats vs. sham-operated group (P = 0.001).
c Significant difference in lesioned pretreated animals with 50mg/kg genistein vs. lesioned group (P = 0.01).
5. Discussion
This study aimed to examine the effects of two different doses of the isoflavone genistein (50 mg/kg and 500 mg/kg) on dopaminergic neurons in a rat model of hemi-parkinsonism. Our findings indicate that genistein at a dose of 50 mg/kg mitigates apomorphine-induced rotational behavior in a 6-OHDA hemi-parkinsonism model by alleviating neuroinflammation and oxidative stress. However, a higher dose of 500 mg/kg failed to protect nigrostriatal neurons from 6-OHDA-induced toxicity and DNA fragmentation. In fact, the higher dose appeared to exacerbate the neurotoxic effects of 6-hydroxydopamine. The 6-OHDA lesion selectively induces the degeneration of dopaminergic neurons in the nigrostriatal pathway, causing an asymmetry in dopamine levels between the brain hemispheres. This imbalance disrupts basal ganglia activity and manifests as biased motor outputs. The degree of rotational behavior following pharmacological stimulation correlates with the extent of dopaminergic depletion and functional impairment in the lesioned hemisphere (14). Apomorphine, a dopamine receptor agonist, elicits contralateral rotations to the lesion side by stimulating supersensitized postsynaptic dopamine receptors in the denervated hemisphere. This behavior reflects the functional dopaminergic imbalance caused by the lesion, with the frequency and direction of rotations serving as quantitative measures of nigrostriatal damage (15). In our experiments, apomorphine administration induced increased contralateral rotations, indicative of upregulated dopamine receptor sensitivity in the lesioned hemisphere. The SOD is a key antioxidant enzyme that reduces oxidative stress by converting superoxide radicals into hydrogen peroxide. In Parkinson's disease models, reduced SOD activity exacerbates oxidative damage in the substantia nigra, impairing dopaminergic signaling and leading to motor deficits such as increased rotational behavior. In this study, the significant increase in SOD activity observed with 50 mg/kg genistein correlates with reduced oxidative stress and improved motor function. Conversely, the decline in SOD activity with the 500 mg/kg dose likely exacerbates oxidative damage, impairing motor outcomes.
Previous studies have demonstrated a dose-dependent increase in SOD and glutathione peroxidase (GPx) levels in peritoneal fluid following genistein treatment in a mouse model of endometriosis. At lower doses (0.13 - 0.26 mg), genistein exhibited antioxidant properties (16). However, higher doses (500 - 1000 mg/kg) increased oxidative stress and induced adverse effects via various cellular mechanisms (17). These findings align with our observations, where a 500 mg/kg dose of genistein failed to produce neuroprotective effects and instead aggravated neurotoxicity. Genistein, an aglycone form of isoflavones, is a well-known phytoestrogen with neuroprotective, antioxidant, anti-inflammatory, and anti-proliferative properties (18). Its structural similarity to estrogen allows it to exhibit both estrogenic and anti-estrogenic effects by interacting with estrogen receptors (19). As our results suggest, the effects of genistein are dose-dependent, with low doses producing protective effects and high doses potentially interfering with neuroprotection. Genistein crosses the blood-brain barrier, and its accumulation in the brain varies with dosage (20, 21). Its non-genomic effects, including tyrosine kinase inhibition and antioxidant activity, are well-documented (22). In this study, genistein at 50 mg/kg exhibited antioxidant and anti-apoptotic effects, consistent with previous findings of its ability to reduce oxidative stress and apoptotic markers at lower doses (14). Notably, high doses of genistein have been associated with undesirable effects mediated through non-estrogenic pathways. These findings align with earlier reports of its failure to induce neuroprotective or anti-inflammatory effects at higher concentrations, such as the 500 mg/kg dose used in this study (23). Genistein's neuroprotective potential has been extensively studied in neurodegenerative disease models. In Parkinson's and Alzheimer's disease models, genistein reduced amyloid-beta toxicity, improved neuronal survival, and enhanced cognitive and motor functions (24). Similarly, its antioxidant defense mechanisms, including the upregulation of SOD and catalase, have been shown to mitigate oxidative stress (13, 25).
In this study, genistein reduced 6-OHDA-induced rotational behavior at 50 mg/kg, likely through enhanced SOD activity and reduced levels of TNF-α, a pro-inflammatory cytokine linked to neuroinflammation and neuronal apoptosis. However, the higher dose of 500 mg/kg failed to reduce TNF-α levels, correlating with persistent neuroinflammation and impaired motor function. These results underscore the dose-dependent relationship between genistein's biochemical and behavioral effects (17). TNF-α, as a pro-inflammatory cytokine, plays a pivotal role in neuroinflammation. Elevated levels of TNF-α are associated with neuronal apoptosis and synaptic dysfunction, contributing to motor impairments in Parkinsonian models (26). In our study, the reduction in TNF-α levels with 50 mg/kg genistein likely reflects diminished neuroinflammation, which may preserve neuronal integrity and improve motor behavior. On the other hand, the lack of significant reduction in TNF-α levels with 500 mg/kg genistein correlates with persistent inflammation and a failure to ameliorate motor deficits. By mitigating oxidative stress and neuroinflammation, genistein at 50 mg/kg appears to protect dopaminergic neurons, leading to improved motor performance. In contrast, the lack of biochemical improvements with the higher dose (500 mg/kg) is consistent with its failure to reduce rotational behavior. This highlights the direct relationship between these biochemical markers and motor function.
In studies involving rats, genistein has demonstrated the ability to inhibit apoptosis in various neuronal contexts (27). For instance, it effectively reduced the expression of pro-apoptotic proteins such as Bax and caspases while enhancing the expression of the anti-apoptotic protein Bcl-2 (27). This modulation leads to a decrease in neuronal cell death, particularly in the hippocampus of ovariectomized rats, where long-term treatment with genistein resulted in a notable reduction in apoptosis rates (28). This may suggest that the beneficial effect of genistein may arise via its antioxidant and anti-inflammatory capacity. However, we cannot rule out the possibility that long-term administration of genistein may inhibit the onset of oxidative stress. Further research studies are required to clarify the exact mechanisms underlying the genistein effects at different doses. We will continue immunohistochemistry techniques to deepen the understanding of the current results.
Notably, the current study focuses on the short-term effects of genistein in a 6-OHDA rat model, which is sufficient to capture acute neuroprotective or neurotoxic effects. However, Parkinson’s disease is a chronic condition, and the therapeutic efficacy of genistein must also be evaluated in long-term paradigms. Prolonged studies would help determine whether the neuroprotective benefits observed with 50 mg/kg genistein persist over time and whether higher doses continue to exert neurotoxic effects. Additionally, long-term studies could reveal potential cumulative effects on motor and non-motor symptoms, as well as mechanisms such as neurogenesis or compensatory pathways. Future experiments incorporating chronic treatment and follow-up will provide deeper insights into the suitability of genistein for long-term therapeutic use.
The findings of this study demonstrate that low-dose genistein (50 mg/kg) exhibits significant neuroprotective effects by improving motor function, reducing oxidative stress, and lowering neuroinflammatory markers in a 6-OHDA rat model of Parkinsonism. In contrast, the higher dose of genistein (500 mg/kg) either fails to produce beneficial effects or exacerbates neurotoxicity, likely due to its dose-dependent impact on oxidative stress pathways. These results emphasize the importance of dose optimization when considering genistein as a potential therapeutic agent for neurodegenerative diseases. Further studies are needed to explore the underlying mechanisms and long-term outcomes of genistein treatment.
5.1. Limitations
While this study provides valuable insights into the dose-dependent effects of genistein on neurodegeneration and motor function in a 6-OHDA rat model of hemi-parkinsonism, several limitations should be considered. The effects of genistein were assessed over a limited period, whereas PD is a chronic condition. Long-term studies are needed to determine sustained effects. Only two doses were tested, limiting insight into intermediate or optimal dosing. Future studies should explore a broader range. While oxidative stress and neuroinflammation markers were measured, additional biomarkers could provide a more comprehensive mechanistic understanding.