1. Background
More than 25% of low back pain is attributable to the sacroiliac joint (SIJ) or hips (1). In a study of 14,552 Medicare patients with SIJ disruption or degeneration, the average direct medical costs per patient over five years amounted to approximately 18,500$ (2). Currently, there is no established standard tool for the diagnosis and management of SIJ pain. Furthermore, existing studies face limitations in using pain intensity measurement scales, such as the Numeric Rating Scale (NRS), for patients with chronic SIJ pain and in reporting complications (3, 4).
This condition is commonly managed through conservative approaches or interventions, including intra-articular (IA) and peri-articular injections, radiofrequency ablation, and arthrodesis (5). The efficacy of SIJ injections with various substances, including corticosteroids, platelet-rich plasma (PRP), prolotherapy, hyaluronic acid, and local anesthetics, has gained attention in recent studies (6-9). The most common injectable option is corticosteroids, which are widely used to relieve SIJ-related pain effectively (10). However, the limited duration of steroid impact on SIJ pain necessitates repeated injections (11, 12), leading to potential systemic side effects, such as inadequate cortisol release from the adrenal glands due to negative feedback, elevated glucose levels, increased systolic blood pressure, and diminished bone density (10, 13-15). As a result, there is a growing demand for other nonsurgical alternatives, such as sacral lateral branch radiofrequency ablation. King et al., in a systematic review regarding the application of radiofrequency ablation for posterior SIJ pain, estimated that 50% of patients experienced a 50% reduction in pain (16). However, challenges in accessing the anterior innervation of the SIJ, combined with the temporary effects of the procedure, limit the application of radiofrequency ablation (16-18).
Platelet-rich plasma provides a rich autologous supply of growth factors. Due to its potential for tissue regeneration, minimally invasive administration, affordable cost, and low risk of hypersensitivity reactions, PRP is widely applied in orthopedics and pain medicine (19).
2. Objectives
Although PRP is widely utilized for musculoskeletal conditions, there is limited evidence regarding its application in SIJ pain (20). This study aims to assess the efficacy of PRP injection in alleviating pain and improving disability in patients with chronic SIJ pain, as well as to evaluate its complications. Conducting this study can enhance the limited existing knowledge about the role of PRP as a treatment modality for chronic SIJ pain.
3. Methods
3.1. Participants
This study is a single-arm, open-label clinical trial conducted at a tertiary care university hospital in Tehran, Iran, from September to November 2024. Eligible participants were patients aged 30 to 80 years, classified as class I or II according to the American Society of Anesthesiologists (ASA) physical status classification. To be included in this study, patients were required to have experienced low back pain for over three months, located below the L5 level, with a pain intensity of 5 or above on the NRS. Additionally, they had to remain unresponsive to conservative treatments, including pain-relief medications and physical therapy. All prior therapies were discontinued prior to the trial, and pain medications were withdrawn at least 48 hours before the injection.
Patients exhibiting signs of nerve root involvement or a history of previous spinal surgery were excluded from this study. Furthermore, any medical conditions that could potentially affect the response to PRP or compromise the safety of the procedure were considered exclusion criteria. These included thrombocytopenia, coagulopathies, systemic or local infections at the injection site or in the surrounding area, cancer, autoimmune disorders, immunodeficiencies, uncontrolled diabetes mellitus, hypertension, and pregnancy. Additionally, individuals who had used non-steroidal anti-inflammatory drugs (NSAIDs) within 48 hours or steroids within 1 month prior to the trial were excluded.
The diagnosis of SIJ pain was established through a comprehensive physical examination by a pain fellow during the first visit. The following provocative tests were performed on each patient: FABER test, thigh thrust, iliac compression, iliac distraction, Yeoman test, Gaenslen’s test, and Fortin finger test. At least three positive tests were required to confirm the diagnosis.
3.2. Platelet-Rich Plasma Processing
Before the intervention, 60 mL of blood was obtained from each patient’s cubital vein and placed in Rooyagen kits containing 6 mL of citrate phosphate dextrose and adenine (CPD-A1). The whole blood underwent a 2-step centrifugation process: The first spin was conducted at 1200 rpm for 15 minutes, followed by a second cycle at 2700 rpm for 5 minutes. This process yielded a final volume of 10 mL of PRP. Sterile conditions were maintained throughout all steps of PRP preparation.
Mishra classified PRPs into four types and two subtypes. Type 1 and type 2 both contain concentrated white blood cells (WBCs) and platelets, which are inactivated in type 1 and activated in type 2. Type 3 and type 4 contain increased levels of inactivated and activated platelets, respectively, but without WBCs. Subtype A has platelet levels more than five times the baseline, but lower than those in subtype B. The PRP we obtained contained highly concentrated WBCs and inactivated platelets at levels at least five times the baseline, categorizing it as type 1A according to this classification (21).
3.3. Procedure
After explaining the risks and benefits of the intervention to each participant, informed consent was obtained. Participants received unilateral or bilateral SIJ injections under ultrasound (US) guidance in the operating room, depending on the side of their symptoms. Under ASA monitoring standards, the patient was placed in a prone position with a thin pillow under the hips. After prepping the skin of the target area and applying drapes, the following steps were undertaken to identify the accurate site for the procedure: (1) A curvilinear ultrasound (US) probe with a frequency range of 2 - 5 MHz was used in a short axis orientation to locate the sacral hiatus along the midline; (2) the probe was maneuvered laterally to identify the lateral border of the sacrum as the initial bony landmark; and (3) the iliac bone was located as the second contour by moving the probe cephalad. Between the two echogenic lines of the iliac and sacral structures, the SIJ was visible as a hypoechoic area.
While continuously visualizing the needle tip on the US screen, a 22-gauge spinal needle was inserted from medial to lateral into the target point of injection at the level of the second sacral foramen and inferior aspect of the joint space. Once the needle was placed properly, 10 mL of PRP was injected. After the procedure, the patients were positioned supine and closely monitored for at least 30 minutes for any changes in their vital signs or any immediate adverse events or complications.
3.4. Data Collection
The patients were followed up at 1 month and 3 months post-intervention. Functional disability and pain intensity were recorded before the procedure, 1 month later during an office visit, and 3 months later through a phone call. In addition, patients were asked about any adverse events or complications.
We used the modified Oswestry disability questionnaire (MODQ) to measure functional disability. This scale provides a percentage of disability ranging from 0% to 100%. The classifications are as follows: Zero percent to 20% as minimal disability, 20% to 40% as moderate disability, and 40% to 60% as severe disability. Scores between 60% and 80% indicate a crippled disability status, while scores of 80% to 100% suggest the individual is bed-bound (22). Baradaran et al. validated this inventory with a Cronbach’s alpha coefficient of 0.69 (23). Pain intensity was measured using a NRS, which ranges from 0 to 10. A score of 0 indicates no pain, while scores under 4 indicate mild pain, 4 to 6 indicate moderate pain, and scores above 6 represent severe pain (24).
3.5. Statistical Analysis
The entire dataset was analyzed using SPSS 23 (IBM Corp., Armonk, NY, USA). Descriptive variables were reported as frequencies and percentages, while numeric values were presented as means and standard deviations (SD). The Shapiro-Wilk test was used to assess the normal distribution of the quantitative variables. A repeated measures analysis of variance (ANOVA) was employed to evaluate changes in pain intensity and functional disability as measured by the NRS and Modified Oswestry Disability Index (MODI) scales during the follow-up periods. P-values less than 0.05 were considered statistically significant.
4. Results
4.1. Participants Characteristics
Sixteen patients were included in the study, with a mean age of 62.81 years (± 15.44). The majority of participants (75%) were female. The demographics and clinical characteristics of the participants are provided in Table 1.
| Variables | Values |
|---|---|
| Age | 62.81 ± 15.44 |
| BMI | 28.61 ± 4.00 |
| Gender | |
| Male | 4 (25.0) |
| Female | 12 (75.0) |
| ASA grade | |
| Grade I | 11 (68.8) |
| Grade II | 5 (31.2) |
| SIJ involvement side | |
| Isolated right | 11 (68.75) |
| Isolated left | 3 (18.75) |
| Bilateral | 2 (12.5) |
Abbreviations: BMI, Body Mass Index; SD, standard deviation; N, number; ASA, American Society of Anesthesiologists; SIJ, sacroiliac joint.
a Values are presented as No. (%) or mean ± SD.
4.2. Evaluation of Pain Intensity and Functional Disability
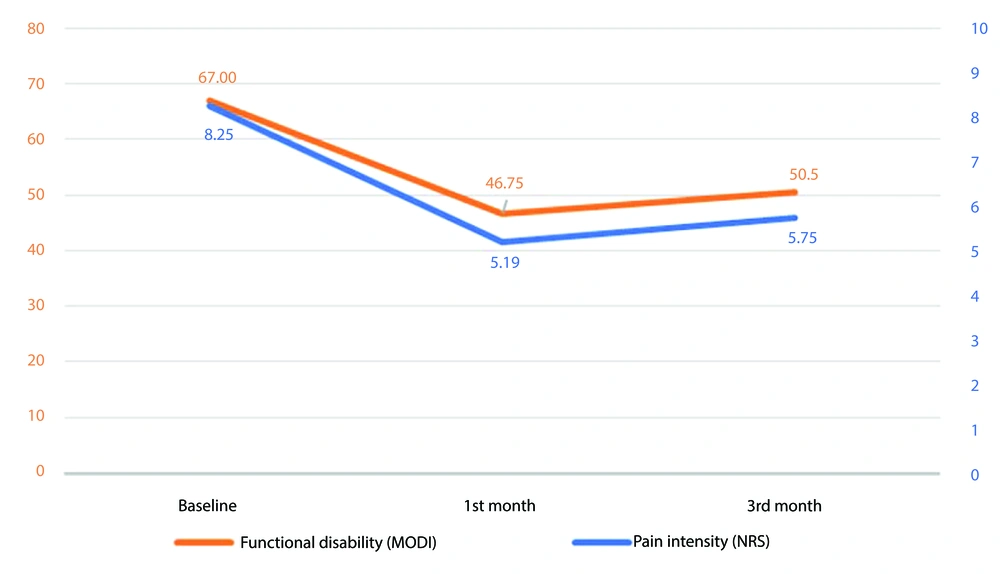
Table 2 shows the results of pain and disability assessments at baseline, one-month, and three-month follow-ups. The mean NRS, as an indicator of pain intensity, was 8.25 ± 1.06 before the injection. At the one-month follow-up, the mean NRS decreased to 5.19 ± 2.66. By the three-month follow-up, the mean NRS score of patients was 5.75 ± 2.54. The overall change in NRS over three months was statistically significant (P < 0.001, Figure 1). Post hoc analysis of NRS scores revealed a significant decline in pain intensity from baseline to both the one-month and three-month follow-ups. These results indicate that the intervention effectively reduced pain intensity in patients, with a notable initial response at the one-month mark, followed by a slight increase at the three-month follow-up.
Abbreviations: NRS, Numeric Rating Scale; MODI, Modified Oswestry Disability Index; SD, standard deviation.
a Values are presented as mean ± SD.
b Significant difference between baseline and first month.
c Significant difference between baseline and third month.
Trends in functional disability and pain intensity over three-month follow-up (P < 0.001). The functional disability measured by Modified Oswestry Disability Index (MODI) (orange line, left Y-axis) and the pain intensity measured by Numeric Rating Scale (NRS) (blue line, right Y-axis) both show a significant reduction from baseline to the first month and remain significantly lower than baseline at the third month, despite a slight increase observed.
In terms of functional disability, the mean MODI was 67.00 ± 8.42, indicating a significant level of disability among patients. The mean MODI scores were 46.75 ± 21.46 at the one-month follow-up and slightly increased to 50.50 ± 19.98 at the three-month follow-up. These results demonstrate a significant reduction in functional disability over the three months, with a P-value of < 0.001 (Figure 1). Post hoc analysis of the MODI scores confirmed a significant decrease in functional disability from baseline to both the one-month and three-month follow-ups. The results suggest that although the intervention improved the functional outcomes of patients from baseline, the slight increase in mean MODI scores from the one-month to the three-month follow-up may necessitate further investigation into the long-term effects of PRP injection.
No substantial adverse reactions, side effects, or complications were reported during the follow-ups. Pain at the injection site immediately after the intervention was the only reported post-procedure complaint.
5. Discussion
In the present study, the efficacy and sustainability of PRP injection in reducing SIJ-related pain and functional disability were assessed, with attention to potential side effects. Although the majority of pain intensity and functional disability improvement occurred within the first month after injection, the PRP effect in improving pain and disability persisted up to three months after the procedure compared to baseline. No major complications were observed during the follow-up.
Navani and Gupta published the practical potential of PRP injection for alleviating SIJ pain in 2016. They conducted IA injections under fluoroscopic guidance in 10 individuals suffering from chronic SIJ pain. The impact of PRP remained significant even one year after the injection (25), which was consistent with our results.
In 2017, Ko et al. further demonstrated the effectiveness of US-guided peri-articular PRP for both functional enhancement and pain relief in four individuals with chronic SIJ-related low back pain. They assessed pain intensity using the NRS and functional disability via the ODI scale at 1-year and 4-year intervals post-injection. The findings revealed improvements in both pain intensity and functional disability at the follow-ups compared to baseline measurements. However, both ODI and NRS scores deteriorated at the second follow-up, consistent with the results of our study (21).
Singla et al., in a randomized clinical trial, compared the impact of steroid versus PRP injection on 40 patients with chronic SIJ-related low back pain. They applied the visual analog scale (VAS) and MODQ to evaluate pain intensity and functional disability, respectively. Their results revealed that PRP reduced pain intensity by at least 50% in 90% of patients three months after the injection, which is similar to our report. However, only 25% of participants in the steroid group demonstrated at least a 50% reduction in pain intensity at the same follow-up point. Additionally, they found that the impact of PRP was noticeable at the 2- and 4-week follow-ups and gradually decreased 3 months after the injection, which is in agreement with our findings (11).
Wallace et al., in a non-randomized trial, revealed the promising effect of PRP injection under a US real-time monitor in 50 patients with SIJ dysfunction-associated pain. They measured pain intensity and functional disability through the NRS and ODI scales, respectively, pre-injection and at 2, 4, 12, and 24 weeks post-injection. The majority of the pain and functional improvement occurred 2 weeks after the injection and slightly tapered during the 6 months after the injection. However, the effect of PRP was still significant regarding pain intensity and functional outcomes after 6 months compared to baseline (26).
In a randomized double-blinded study, Chen et al. evaluated the efficacy of IA PRP injections versus steroid injections in 26 patients with block-confirmed SIJ pain. Analysis of pain intensity yielded greater steroid impact in terms of pain intensity than PRP at first-, third-, and sixth-month evaluations. Moreover, the steroid group showed a more substantial response in mitigating functional disability compared to the PRP group at one- and three-month post-injection. However, this difference was not significant at the sixth-month follow-up. Furthermore, steroid injection led to a 50% pain decrease in 80% and 70% of the patients in the first and third months, respectively. After one and three months, 21.4% of individuals in the PRP group experienced 50% pain reduction (27).
In agreement with our study, several papers reported no serious complications following PRP injection for musculoskeletal issues and SIJ-related pain, which makes PRP administration a minimally invasive and safe method (6, 11, 21, 25, 28, 29).
Despite previous studies by Navani and Chen, who monitored direct IA injection into the SIJ space (25, 27), we did not confirm direct IA injection via contrast. Singla et al. and Wallace et al.’s (11, 26) PRP injections were also based on US guidance; thus, their injections might have involved both intraarticular and periarticular areas. This difference in injection techniques may influence the comparability of the studies. Future studies are suggested to clarify the efficacy of intraarticular versus periarticular PRP injections.
Although this study contributes valuable information about the efficacy of PRP injections for chronic SIJ pain, several limitations should be considered. The major limitation of this study is the single-arm design and the absence of a control group. Additionally, only 16 patients participated, which provides a small sample size. While explanatory studies with samples between 10 and 15 participants can show efficacy ranging from 20 to 30 percent, these concerns necessitate future randomized controlled trials with a larger sample size to ensure the reliability of the results. The follow-up interval in our study was limited to 3 months; therefore, longer follow-up is essential to investigate the sustainability of the PRP effect. Although blockade via injection of anesthetics into the joint is considered the confirmatory test for SIJ pain, our diagnosis was based on the presence of at least three positive provocative tests. According to Laslett et al., three or more positive results from the six provocative tests yielded 94% sensitivity and 78% specificity for detecting SIJ pain (30).
5.1. Conclusions
This study highlights the capacity of PRP injections in the management of SIJ-related pain and functional disability. Our findings indicate that although the potential impact of the injection was observed within 1 month post-injection, the effects were significant up to 3 months compared to baseline measurements, suggesting its potential as a minimally invasive therapeutic option for chronic SIJ pain. This modality could serve as an alternative for patients who are unresponsive to conventional therapies or wish to avoid more invasive surgical interventions.