1. Background
Parkinson's disease (PD) is a progressive neurological disorder that causes damage to dopamine neurons in the substantia nigra of the brain (1). The PD is a neurodegenerative disease that is more prevalent in older adults. It is the most common movement disorder and the second most common disease of neuronal degeneration. Common disorders in this disease include motor, cognitive, and mitochondrial disorders (2-4). Factors involved in the development of PD include aging, environmental factors, and genetic factors. With aging, the amount of dopamine and the number of its receptors in the basal ganglia permanently decrease, accelerating the onset of PD symptoms. Environmental factors include exposure to pesticides and metals, which are harmful through mechanisms such as induction of reactive oxygen species production, changes in mitochondrial metabolism, and alterations in the redox cycle. Genetic factors include genetic changes such as PARK1 to PARK16, which can play an effective role in the development of PD (5-8).
The main symptoms of PD include tremor, abnormal slowness of movements, impaired balance and gait, muscle rigidity, tremor at rest, akinesia or difficulty in initiating movements, decreased spontaneous movements, bradykinesia or slowness of movements, poor balance, decreased dependent movements, and changes in facial expressions during speaking, enuresis, and constipation (9, 10). Additionally, the risk of dementia in PD is about 6 to 8 times that of control groups of the same age and sex, with a long-term prevalence of approximately 80% (11). Voice disorders are also prevalent, including hypokinetic dysarthria, disorders of voice, articulation and rate, as well as reduced loudness, vowel centralization, and imprecise consonants (10, 12, 13). Besides physical problems, these patients experience complications such as depression, anxiety, symptoms of mental distress, and apathy, each of which can affect the patient's quality of life (14, 15). Currently, there is no easy and accessible definitive treatment for PD. Consequently, PD poses many challenges for caregivers at home or in the hospital. If the caregiver is the patient's spouse, they are often forced to change roles and must perform the roles of their spouse long-term (16, 17). In the case of hospitalization, care should aim to improve the health of these patients and prevent complications resulting from hospitalization (18-21). One way to improve the health of hospitalized patients is to provide medical and nursing care in the ICU, which is why ventilator-related indicators are essential considerations (22-25).
2. Objectives
Considering the complications caused by PD, this study was conducted to determine the clinical outcomes of patients with PD who are ventilator-dependent.
3. Methods
3.1. Study Design
This study is part of a registry data set. The Iran ICU Registry (IICUR) is a Persian ICU-based registry that was launched in 2018 through a collaboration with the Australian and New Zealand Intensive Care Society (ANZICS). The IICUR was approved by the Ethics Committee of Shiraz University of Medical Sciences (ethics number: IR.SUMS.REC.1397.559) and is recognized by the Iran Ministry of Health as the first and only registry of adult ICUs in Iran.
3.2. Study Population
This case-control study was conducted in the ICU of Razi Hospital in Ilam, Iran, involving a group of hospitalized patients with underlying PD (16 patients) and a control group (36 patients).
3.3. Inclusion and Exclusion Criteria
Intubated patients who were definitively diagnosed with PD based on their clinical records and a neurologist's assessment were included in the study. Patients meeting these criteria were assigned to the case group, while those without PD who met the inclusion criteria were assigned to the control group.
3.4. Research Method
Researchers visited the ICU ward daily, extracted patient information from medical records based on clinical symptoms and laboratory test results, and recorded it in a checklist.
3.5. Data Gathering
Researchers completed patient data using a registry checklist. The checklist included questions on age (mean ± SD), gender, disease duration, BMI, APACHE III score, and past medical history (diabetes, renal dialysis, immunosuppression, chronic respiratory disease, leukemia/myeloma, and chronic cardiovascular disease). Additionally, it recorded the length of stay (days) in the ICU or hospital and mortality (in the ICU or hospital).
In this study, indicators related to intubated patients dependent on ventilators were assessed, including synchronized intermittent mandatory ventilation (SIMV), PaO2 (partial pressure of arterial oxygen), FiO2 (fraction of inspired oxygen), delirium (%), and duration of mechanical ventilation (days). Laboratory parameters measured included pH, PaO2/FiO2 ratio, PaCO2 (mmHg), HCO3- (mmol/L), Hb (g/L), No. (%), BUN (mmol/L), Cr (µmol/L), Na+ (mmol/L), and K+ (mmol/L).
3.6. Data Analysis
After data collection, the information for this study was entered into SPSS version 16 and analyzed using descriptive tests, including frequency analysis (number and percentage). Additionally, the P-value was reported at a significance level of less than 5% (P < 0.05).
4. Results
In this study, 16 patients with PD were placed in the case group, and 36 patients with other neurological disorders hospitalized in the ICU were placed in the control group. The results showed that 18.8% of patients with PD had a history of diabetes, 6.3% had a history of dialysis problems, 12.5% had immunosuppressed diseases, 31.3% had respiratory disease, and 18.8% had chronic cardiovascular disease (Table 1).
| Variables | SIMV; Case (N = 16) | SIMV; Control (N = 36) | P-Value |
|---|---|---|---|
| Gender | 0.28 | ||
| Male | 10 (62.5) | 14 (53.8) | |
| Female | 6 (37.5) | 12 (46.2) | |
| Diabetes | 0.58 | ||
| Yes | 3 (18.8) | 4 (15.4) | |
| No | 13 (81.3) | 22 (84.6) | |
| Renal dialysis | 0.73 | ||
| Yes | 1 (6.3) | 2 (7.7) | |
| No | 15 (93.8) | 24 (92.3) | |
| Immunosuppressed | 0.61 | ||
| Yes | 2 (12.5) | 4 (15.4) | |
| No | 14 (87.5) | 22 (84.6) | |
| Chronic respiratory disease | 0.003 | ||
| Yes | 5 (31.3) | 3 (11.5) | |
| No | 11 (68.8) | 23 (88.5) | |
| Leukemia/myeloma | 0.038 | ||
| Yes | 2 (12.5) | 1 (3.8) | |
| No | 14 (87.5) | 25 (96.2) | |
| Chronic cardiovascular | 0.07 | ||
| Yes | 3 (18.8) | 8 (30.8) | |
| No | 13 (81.3) | 18 (69.2) | |
| APACHE III score | 15.25 ± 1.61 | 15.96 ± 2.04 | 0.20 |
| Height (cm) | 171.18 ± 9.29 | 169.96 ± 5.61 | 0.02 |
| Weight (kg) | 67.68 ± 11.24 | 73.23 ± 13.16 | 0.33 |
| Length of stay in ICU | 17.31 ± 5.09 | 11.42 ± 3.76 | 0.84 |
| Length of stay in hospital | 21.68 ± 3.28 | 16.69 ± 3.96 | 0.99 |
| Days on ventilator | 13.06 ± 4.28 | 8.88 ± 3.25 | 0.15 |
Demographic Characteristics of Patients in the Control and Case Groups a
The findings in Table 2 show the comparison of clinical and laboratory indicators between the PD group and the control group. According to the findings, there was no significant difference in all variables, except for PaO2/FiO2 and HCO3- (mmol/L), between the two groups (P > 0.05).
| Variables | SIMV; Case (N = 16) | SIMV; Control (N = 36) | P-Value |
|---|---|---|---|
| PH | 7.74 ± 0.15 | 7.75 ± 0.13 | 0.81 |
| PaCO2 (mmHg) | 42.75 ± 1.73 | 43.23 ± 2.1 | 0.42 |
| PaO2 /FiO2 | 125.37 ± 9.81 | 134.65 ± 3.58 | 0.000 |
| HCO3- (mmol/L) | 23.87 ± 0.95 | 23.8 ± 1.95 | 0.04 |
| Hb (gr/dL) | 13.37 ± 1.14 | 13.03 ± 1.56 | 0.08 |
| BUN (mg/dL) | 39.25 ± 1.84 | 39.07 ± 3.01 | 0.08 |
| Cr (mg/dL) | 0.975 ± 0.28 | 0.93 ± 0.27 | 0.2 |
| WBC (cell/L) | 17.5 ± 0.89 | 16.34 ± 1.19 | 0.2 |
| Na+ (mEq/L) | 141.68 ± 4.06 | 140.61 ± 4.44 | 0.8 |
| K+ (mEq/L) | 3.84 ± 0.31 | 4.11 ± 0.40 | 0.15 |
Comparison of Mean ± SD Laboratory Measures of Patients in the Control and Case Groups
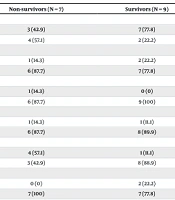
The findings in Table 3 show the comparison of the variables studied between non-survivors and survivors. According to the findings, factors such as renal dialysis, chronic respiratory disease, and leukemia/myeloma were associated with patient mortality (P < 0.05). Additionally, the APACHE III score was associated with mortality (P = 0.001).
| Variables | Non-survivors (N = 7) | Survivors (N = 9) | P-Value |
|---|---|---|---|
| Gender | 0.15 | ||
| Male | 3 (42.9) | 7 (77.8) | |
| Female | 4 (57.1) | 2 (22.2) | |
| Diabetes | 0.44 | ||
| Yes | 1 (14.3) | 2 (22.2) | |
| No | 6 (87.7) | 7 (77.8) | |
| Renal dialysis | 0.01 | ||
| Yes | 1 (14.3) | 0 (0) | |
| No | 6 (87.7) | 9 (100) | |
| Immunosuppressed | 0.72 | ||
| Yes | 1 (14.3) | 1 (11.1) | |
| No | 6 (87.7) | 8 (89.9) | |
| Chronic respiratory disease | 0.01 | ||
| Yes | 4 (57.1) | 1 (11.1) | |
| No | 3 (42.9) | 8 (88.9) | |
| Leukemia/myeloma | 0.002 | ||
| Yes | 0 (0) | 2 (22.2) | |
| No | 7 (100) | 7 (77.8) | |
| Chronic cardiovascular | 0.10 | ||
| Yes | 2 (28.6) | 1 (11.1) | |
| No | 5 (71.4) | 8 (88.9) | |
| APACHE III score | 16.57 ± 1.61 | 14.22 ± 0.44 | 0.001 |
| Height (cm) | 174.57 ± 9.27 | 168.55 ± 8.93 | 0.94 |
| Weight (kg) | 65.28 ± 11.68 | 69.55 ± 11.2 | 0.94 |
| Length of stay in ICU | 19.14 ± 5.72 | 15.88 ± 4.28 | 0.41 |
| Length of stay in hospital | 22.57 ± 2.87 | 21 ± 3.57 | 0.33 |
| Days on ventilator | 14.57 ± 3.9 | 11.88 ± 4.4 | 0.46 |
Comparison of Mean ± SD Survivors of Patients in Case Groups a
5. Discussion
This study aims to determine the respiratory and hemodynamic stability of ventilator-dependent patients with PD. In this study, most patients were elderly, and 62.5% were male. In the study by Lieberman et al., which included 630 PD patients, it was shown that most patients were male and elderly (26). Similarly, in the retrospective cohort study by Shahrestani et al., the mean age of the patients was 76.3 years, and 55.9% were male (27). These findings are consistent with the results of this study. The results showed that 18.8% of patients with PD had a history of diabetes, 6.3% had a history of dialysis problems, 12.5% had immunosuppressed diseases, 31.3% had respiratory disease, and 18.8% had chronic cardiovascular disease. In the cohort study by Réa-Neto et al., it was shown that the reasons for hospitalization of PD patients included neurological diseases (15.2%), abdominal issues (2.6%), cardiological issues (3.5%), respiratory issues (3.9%), renal/metabolic issues (3%), trauma (17.3%), elective surgery (17.7%), and sepsis (36.8%). There was a significant difference between the cause of hospitalization in the PD group and the non-PD group (P = 0.001) (28).
In this study, the length of stay in the ICU was 17.31 (SD 5.09) days, the length of stay in the hospital was 21.68 (SD 3.28) days, and the days on a ventilator were 13.06 (SD 4.28) days. The values in the control group did not differ significantly from the case group (P > 0.05). In the study by Paul et al., the duration of ICU stay in the PD group was 5.9 days, and the duration of ventilation was 3.8 days (19), which are lower than the results reported in this study. In the study by Guldager et al., which compared physiological variables in PRVC and VC modes, it was shown that in PRVC modes, the PIP was 20, MAIP was 10, PaO2 was 98, PaCO2 was 43, pH was 7.38, and MAP was 76. In VC modes, the PIP was 24, MAIP was 10, PaO2 was 96, PaCO2 was 43, pH was 7.38, and MAP was 77. There was a significant difference between the PIP values in PRVC and VC modes (P = 0.001) (29).
In the study by Luo et al., 40 intubated patients with ARDS were included, and the SIMV + PS device mode was evaluated. The results showed that in the survivors group, the status was Hb = 104, percentage of neutrophils = 88.3, ALB = 27.6, PCT = 3.43, FiO2 = 58, PaO2/FiO2 = 135.1, pH = 7.4, and PaCO2 = 38.9. In the non-survivors group, the status was Hb = 110.9, ALB = 28.3, PCT = 11.47, PaCO2 = 44.1, and FiO2 = 71 (30). In the study by Alikiaii et al., in the group of patients with ARDS in the SIMV group, the status was Cr = 1.1, K = 4.2, Na = 141.5, and HCT = 36.6. In the ASV group, the status was Cr = 1.5, K = 4.3, Na = 141.1, and HCT = 29.6 (31).
Chronic illness, ICU hospitalization, and ventilator dependency are factors affecting changes in patients' laboratory parameters. Consequently, these parameters are disrupted, as reflected in the results of this study. Among the limitations of this study is the small sample size, as the number of hospitalized patients diagnosed with PD was low. Therefore, it is recommended that studies be conducted with a larger sample size in patients with PD.
5.1. Conclusions
Given the differences in clinical indicators between patients with PD and the control group, it is suggested that these differences be considered in providing clinical care to these patients.