1. Background
Melatonin, a hormone known for its role in regulating sleep, has demonstrated efficacy in promoting sleep onset and improving sleep quality while producing fewer side effects compared to traditional sleep medications (1). Recent studies have highlighted melatonin's potential beyond sleep regulation, particularly in modulating stress and sympathetic activity. Its interaction with various receptor systems, including adrenergic and opioidergic receptors, suggests a possible role in attenuating the physiological stress responses associated with anesthesia and surgery (2, 3). By reducing anxiety and stabilizing hemodynamics, melatonin may help counteract the harmful effects of sympathetic hyperactivity, which are particularly pronounced during procedures like laryngoscopy and intubation. The physiological response to intubation, which begins immediately, can result in serious cardiovascular complications such as myocardial ischemia, arrhythmias, and cerebrovascular events, especially in patients with underlying hypertension or ischemic heart disease (4).
To mitigate these complications, various medications have been utilized. Addiction introduces additional complexities in anesthesia practice. Patients with substance use disorders, particularly those addicted to μ-opioid receptor agonists, often exhibit altered responses to anesthetic agents, requiring higher doses to achieve the desired effects. Furthermore, these patients are more likely to experience exaggerated hemodynamic changes following laryngoscopy, increasing the risk of complications. Conversely, excessive anesthetic use can lead to hemodynamic instability and prolonged recovery times, presenting a significant challenge for anesthesiologists (5-7).
Emerging evidence suggests that melatonin premedication can significantly reduce the induction dose of propofol and decrease the intraoperative requirements for isoflurane, highlighting its potential benefits in anesthesia management (8-11). Despite these promising findings of the positive effects of melatonin on anesthesia, there is no prior study evaluating the effect of melatonin premedication on patients with μ-receptor agonist addiction, which represents a critical gap in current knowledge.
2. Objectives
In this study, we aim to investigate the effects of melatonin on drug consumption and hemodynamic responses following laryngoscopy in patients with μ-receptor agonist addiction undergoing lumbar spine surgery. This research seeks to address the existing knowledge gap and contribute to the growing body of evidence supporting melatonin's potential role in improving anesthetic outcomes and reducing perioperative complications in this vulnerable patient population.
3. Methods
A triple-blind prospective clinical trial was conducted at Loghman Hakim Hospital from December 2024 to February 2025. The study received approval from the Ethics Committee of Shahid Beheshti University of Medical Sciences, endorsing the trial protocol as a prospective randomized clinical trial (ethics approval code: IR.SBMU.RETECH.REC.1403.432, approval date: October 27, 2024). Additionally, the clinical trial was registered with the Iranian Registry of Clinical Trials (registration code: IRCT20210415050983N9, approval date: November 18, 2024), ensuring compliance with established research standards. The protocol adhered strictly to the ethical principles outlined in the Declaration of Helsinki. All participants were fully informed about the research goals and objectives, and written consent was obtained from each individual.
Participants included patients aged 18 to 60 years with a history of drug addiction, who had been taking at least 1 mg or its equivalent of a μ-receptor agonist daily and continuously for a minimum of six months, provided they experienced withdrawal symptoms if the medication was not taken. Candidates for lumbar spine surgery at Loghman Hakim Hospital were required to be normothermic prior to the operation. Body temperature was measured using a tympanic thermometer (brand: Covidien), and eligible patients were required to have a temperature ranging from 36.8°C to 37.2°C upon entering the study. Furthermore, the study only enrolled patients classified as American Society of Anesthesiology (ASA) I or II.
After consenting to participate, patients were classified according to age and sex. Each patient was then randomly assigned to either the melatonin group or the placebo group (control group). Exclusion criteria included: Difficult airway, Body Mass Index (BMI) greater than 30 kg/m², use of anti-anxiety, sedative, antipsychotic, or antiepileptic medications, presence of sleep disorders, hiatus hernia, gastroesophageal reflux, known allergies to melatonin, and a history of coronary artery disease.
Randomization was performed using R software, which generated 10 random blocks, each consisting of 5 individuals assigned to the intervention group and 5 individuals assigned to the control group. The order of these randomly selected individuals was determined by the computer, and they were assigned to their respective groups in that same order. Once a block was completed, a new block of 10 individuals was created, and this process continued until the final sample size was reached. Individuals not involved in the trial generated the allocation, enrolled patients, and assigned participants to the intervention. Forms labeled with numbers 1 and 2 were placed inside sealed envelopes to facilitate random grouping of the patients. The patients received these envelopes without any knowledge of their group assignments. Upon opening the envelopes, the appropriate drug or placebo, which had been prepared in advance, was administered to the patient based on their group. The clinical caregiver, the evaluator responsible for recording the results, and the person analyzing the data were all blinded to the group assignments.
Addicted patients scheduled for lumbar spine surgery were randomly assigned to either the melatonin group or the placebo group, with 50 participants in each group. The melatonin group received two melatonin tablets (3 mg each), and the placebo group received two placebo tablets, administered 120 minutes before induction. Both pills were contained within foils. Once allocated, a postgraduate student not involved in the trial unsealed the foils and handed the pills to a nurse for administration. Both the nurse and the student were unaware of the allocations.
Hemodynamic parameters were recorded during induction and for 15 minutes after intubation. The total induction dose of propofol (B. Braun Medical) and any adverse effects of melatonin were also documented. Both groups received midazolam 0.03 mg/kg, fentanyl (Caspin Company, Rasht, Iran) 2 - 3 mcg/kg, propofol 1.5 - 2 mg/kg, and cis-atracurium (Caspin Company, Rasht, Iran) 0.2 mg/kg for anesthesia induction. Propofol infusion was maintained at a rate of 100 - 300 mcg/kg during the procedure, with the exact dose criterion for propofol infusion being a Bispectral Index (BIS) of 40 - 60. During surgery, 10 mg of intravenous morphine was administered to all patients.
The primary objective of this study was to maintain hemodynamic parameters within normal ranges. To achieve this, systolic blood pressure (SBP), diastolic blood pressure (DBP), heart rate (HR), and BIS were measured before, during induction, and after intubation. Additionally, the duration of the surgery was recorded. The secondary aim was to compare the amount of administered propofol between the groups. Consequently, the total amount of administered propofol was recorded.
In the event of any complications, medical personnel provided prompt treatment, and the research team covered all expenses. Participants could withdraw from the study at any stage if they were dissatisfied. The individual performing the statistical analysis was blinded to the patient groupings, maintaining a one-sided blinding.
The sample size was calculated using the following formula, based on the results of similar studies and considering a 10% dropout rate. The significance level (α) was set at 0.05 for a 95% confidence interval, and the statistical power (β) was 0.80. To increase precision, the calculated sample size was increased by 50%, resulting in a final sample size of 50 patients per group (100 patients in total).
To minimize the impact of confounding variables, a blinding process was implemented for both the data analyst and the data collector to avoid bias in data interpretation and collection. The number of participants was limited to create a controlled environment, enabling the collection of high-quality data. Robust statistical methods were utilized to account for confounding variables, enhancing the validity and accuracy of the results.
Statistical analysis was conducted using RStudio. A descriptive analysis was performed, and the normality of the distribution of measured variables was evaluated using the Shapiro-Wilk test. Depending on the distribution of the variables, either repeated measures ANOVA or the Friedman test was applied, along with independent t-tests or the Mann-Whitney U test. A P-value of less than 0.05 was considered statistically significant.
4. Results
4.1. Patients’ Characteristics
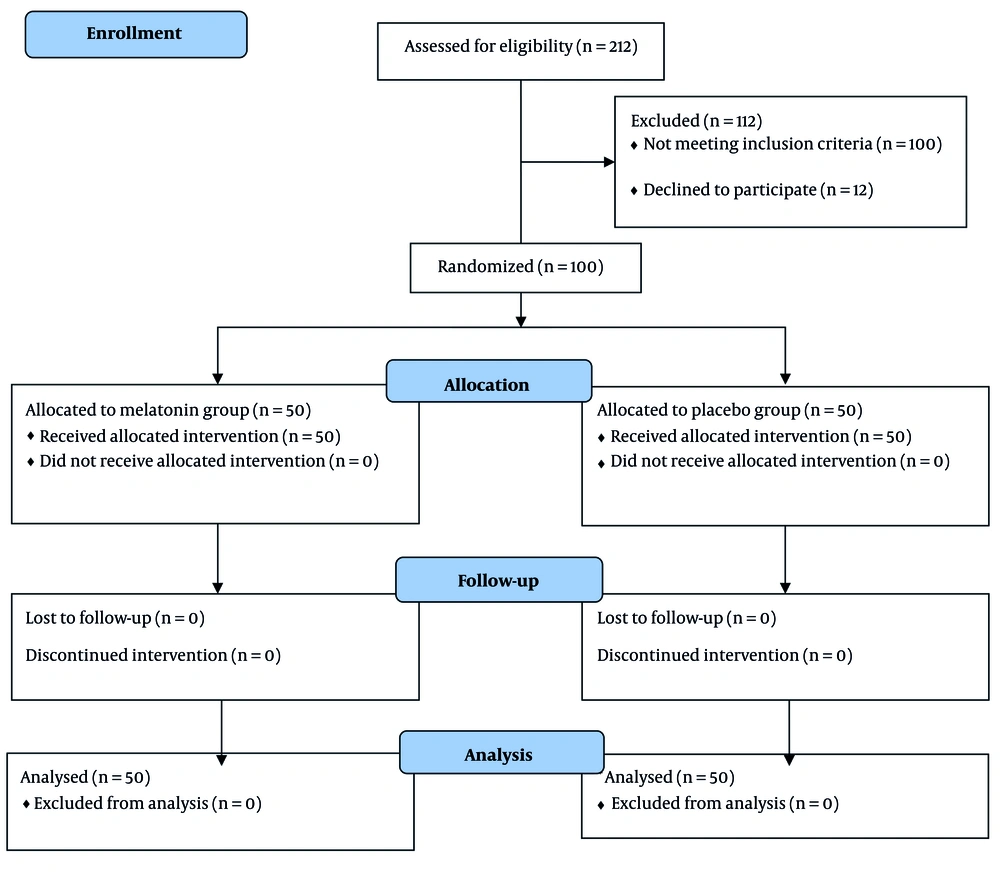
During the three-month recruitment period, 100 patients were enrolled in the study, comprising 38 females and 62 males, with a mean age of 41.79 ± 13.88 years. Each group consisted of 50 patients (Figure 1). Fortunately, no adverse events occurred, allowing all participants to be analyzed until the end of the trial.
In the control group, seventeen females (34%) were included, while in the group that received melatonin, 21 females (42%) were included. The characteristics of the included patients are illustrated in Table 1.
| Variables | Melatonin | Control | P-Value |
|---|---|---|---|
| Age | 41.88 ± 12.26 | 41.7 ± 13.22 | 0.9487 |
| Weight | 71.02 ± 13.19 | 69.32 ± 11.33 | 0.491 |
| SBP | 125.64 ± 8.8 | 124 ± 17.78 | 0.6651 |
| DBP | 84.28 ± 5.04 | 84.46 ± 4.2 | 0.8466 |
| HR | 73.92 ± 8 | 73.44 ± 6.35 | 0.7405 |
| BIS | 96.64 ± 0.85 | 94.28 ± 4.9 | 0.326 |
Abbreviation: BIS, Bispectral Index; SBP, systolic blood pressure; DBP, diastolic blood pressure; HR, heart rate.
a Values are expressed as mean ± SD.
b After conducting the Shapiro-Wilk test, it was revealed that all the variables had normal distribution (P-value > 0.05).
As illustrated in Table 1, there was no statistically significant difference between the two groups regarding basic characteristics or hemodynamic variables before induction or intubation.
4.2. Follow-up Evaluations
Hemodynamic variables, including HR, SBP, DBP, and BIS, were assessed at three consecutive time points: Before induction, during induction, and at the time of intubation. All variables showed a significant increase over time in both groups (P < 0.05).
4.3. Comparing the Results of Two Groups at Each Time Point
The independent t-test was used to assess the differences between the two groups at each time point. The results are presented in Table 2.
| Variables | Melatonin | Control | P-Value |
|---|---|---|---|
| SBP at the time of induction | 93.24 ± 8.97 | 96.08 ± 7.08 | 0.021 |
| SBP at the time of intubation | 128.64 ± 3.86 | 148.78 ± 5.29 | 2 × 10-16 |
| DBP at the time of induction | 62.14 ± 7.45 | 65.9 ± 7.103 | 0.01128 |
| DBP at the time of intubation | 66.84 ± 9.95 | 110 ± 7.04 | 2.2 × 10-16 |
| HR at the time of induction | 99 ± 7.07 | 98.52 ± 8.75 | 2.2 × 10-16 |
| HR at the time of intubation | 106.52 ± 8.75 | 122.38 ± 8.33 | 4.51 × 10-15 |
| BIS at the time of induction | 46.44 ± 3.84 | 48.78 ± 5.44 | 0.008335 |
| BIS at the time of intubation | 54.84 ± 3.16 | 63.4 ± 5.65 | 1.84 × 10-13 |
| Amount of consumed propofol | 2121.63 ± 672.72 | 1765 ± 643.74 | 0.013 |
Abbreviation: BIS, Bispectral Index; SBP, systolic blood pressure; DBP, diastolic blood pressure; HR, heart rate.
a Values are expressed as mean ± SD.
As shown in Table 2, all hemodynamic variables in the group that received melatonin were lower than those in the control group. This suggests that hemodynamics in addicted patients who received melatonin were more stable. Additionally, the BIS score was significantly higher in the group that received melatonin. As demonstrated in Table 2, a higher level of anesthesia was achieved with lower propofol consumption in the melatonin group.
5. Discussion
In this trial, we included 100 patients addicted to μ-receptor agonists who were candidates for lumbar spine surgery. We concluded that patients who received premedication with 6 mg of melatonin had lower SBP, DBP, and HR during induction and intubation. Likewise, the depth of anesthesia was greater in the melatonin group.
Melatonin, often referred to as the pineal hormone, is a neuroendocrine hormone that plays a crucial role in regulating various biological rhythms, particularly the sleep-wake cycle. Biochemically, it is classified as an indole, derived from the amino acid tryptophan. This hormone is primarily synthesized in the pineal gland, a small endocrine gland located in the brain. The discovery of melatonin dates back to 1959 when Dr. Lerner and his team successfully extracted it from the pineal glands of cattle. Since then, extensive research has been conducted to understand its physiological functions and mechanisms of action. Melatonin is notable for its relatively short biological half-life, typically ranging from a few minutes to an hour, necessitating careful consideration of dosage and timing when used as a supplement (1).
Following oral administration, melatonin reaches its highest plasma concentration within an hour. The plasma levels decline in two phases, characterized by half-lives of 2 minutes and 20 minutes. A common dosage (ranging from 1 to 5 mg) results in melatonin concentrations that are 10 to 100 times higher than the natural peak observed at night, with levels returning to baseline within 4 to 8 hours (12).
There has been no previous study assessing the effect of melatonin on the hemodynamics and anesthesia of patients addicted to μ-receptor agonists. However, various studies have evaluated the effect of melatonin premedication on hemodynamics and the depth of anesthesia.
Gupta et al. conducted a clinical trial involving 60 patients undergoing elective surgical procedures. The patients were divided into two groups: One group received 6 mg of melatonin, while the other served as the control. The results indicated that the hemodynamic variables in the melatonin group increased insignificantly, whereas the increase in the control group was significant. Additionally, patients in the melatonin group exhibited lower HRs and blood pressure (13). Similarly, Kumar et al. demonstrated that premedication with 6 mg of melatonin attenuated the rise in HR, SBP, and DBP during intubation (11). Likewise, we showed that melatonin reduces hemodynamic responses during induction and intubation.
Turkistani et al. assessed 45 patients categorized into three groups: Those who received a placebo, those who received 3 mg of melatonin, and those who received 5 mg of melatonin. Propofol was then administered until the BIS reached 45. The amount of propofol administered to the placebo group was significantly higher compared to the other two groups. Additionally, anxiety scores were higher in the placebo group. There was no difference in the time spent in the recovery room among the groups (10).
The results align with the study conducted by Norouzi et al. (9), which included 88 patients undergoing abdominal surgery. The patients were divided into two groups: One group received 3 mg of melatonin, while the other received a placebo. The mean arterial pressure (MAP) was lower in the melatonin group. Similar to the findings of Turkistani et al. (10), anxiety scores were also lower in the melatonin group, and the amount of propofol administered was reduced in this group as well. There was no significant difference between the two groups regarding orientation in the recovery room (9).
Unfortunately, in the current study, we did not assess the level of sedation and anxiety scores in the included individuals. However, we also concluded that a lower amount of propofol was required in the melatonin group.
Most studies indicated that MAP was lower in patients who received melatonin as a premedication. However, Rajan et al. found that melatonin significantly reduced MAP only before and after the induction of general anesthesia. At other time points, MAP levels in both groups were similar (8).
A systematic review conducted by Anderson et al. included 24 studies involving 1,794 patients who received melatonin during the perioperative period. In 21 of these studies, the effects of melatonin were compared to those of a placebo. The meta-analysis revealed that melatonin significantly reduced postoperative pain and anxiety scores (14).
5.1. Clinical Implications
The findings of this study are particularly relevant for anesthetic management in opioid-dependent patients, who often present challenges in achieving stable hemodynamics and adequate anesthesia depth. The ability of melatonin to mitigate cardiovascular fluctuations and reduce anesthetic drug requirements suggests that it could be a valuable adjunct in perioperative care. Its use may potentially lead to improved patient outcomes, reduced anesthetic drug consumption, and a lower incidence of hemodynamic complications.
5.2. Limitations
The study identified several limitations that could affect the interpretation and applicability of the results. Notable omissions include the lack of a comparative analysis of different melatonin doses, timing of administration, sedation and anxiety assessments, psychoanalytic tests post-surgery, and measurements of extubation responses and plasma norepinephrine levels. These gaps highlight critical areas for future research to better understand the effects of melatonin in the perioperative setting.
5.3. Conclusions
In conclusion, this study demonstrated that premedication with melatonin led to a significant reduction in SBP, DBP, and HR during both the induction and intubation phases in patients addicted to μ-receptor agonists. Additionally, patients who received melatonin experienced a deeper level of anesthesia while consuming a lower amount of propofol.