1. Background
Spinal anesthesia involves the intrathecal injection of a local anesthetic to temporarily block nerve conduction, facilitating surgical procedures. It is frequently used in orthopedic surgeries due to its favorable profile, including reduced respiratory and cardiac depression, enhanced tissue perfusion, and extended postoperative analgesia (1). Among available agents, bupivacaine remains a widely preferred local anesthetic for spinal anesthesia, especially in obstetric and postoperative settings (2). Nonetheless, its use can be associated with adverse effects such as post-dural puncture headache and, in rare cases, neurological complications (3, 4). To improve the quality and duration of anesthesia while minimizing adverse effects, adjuvants such as opioids (e.g., fentanyl) and vasoconstrictors like epinephrine are often combined with bupivacaine (5-7). Both epinephrine and fentanyl are among the most commonly studied intrathecal additives, with evidence supporting their efficacy across a variety of surgical procedures, including cesarean section, laparotomy, and joint replacement surgeries (8-10). Bupivacaine is typically administered in concentrations of 0.25%, 0.5%, or 0.75%, offering reliable sensory-motor blockade and prolonged action (11, 12). However, its use may be accompanied by side effects such as nausea, vomiting, shivering, hypotension, seizures, and, in rare cases, cardiac arrest (13). Caution is also advised when it is used in patients taking anticoagulants, antidepressants, or ergot-derivative medications due to potential drug interactions (14).
2. Objectives
Evidence suggests that epinephrine prolongs the duration of analgesia with a relatively low incidence of adverse events (15, 16), while fentanyl enhances intraoperative and early postoperative analgesia but carries risks of respiratory depression and hemodynamic instability, including hypotension (17-22). Although both epinephrine and fentanyl are widely used as intrathecal adjuvants to bupivacaine, their comparative effects in orthopedic surgeries — particularly on motor block duration, hemodynamic responses, and postoperative pain — remain underreported. Therefore, the primary objective of this study was to conduct a comparative analysis of these two commonly used adjuvants in spinal anesthesia, epinephrine and fentanyl, when combined with bupivacaine, focusing on their efficacy, safety, and overall impact on clinical outcomes.
We aimed to evaluate the duration and quality of anesthesia by comparing sensory and motor blockade between the bupivacaine-epinephrine and bupivacaine-fentanyl groups, as well as to assess postoperative pain control over a five-hour period to determine which combination provides more sustained analgesia. Additionally, intraoperative and postoperative hemodynamic parameters — including blood pressure, heart rate (HR), and oxygen saturation — were monitored to evaluate cardiovascular stability and identify any adverse fluctuations. We also recorded the frequency of complications such as hypotension, bradycardia, respiratory depression, nausea, vomiting, pruritus, and shivering to compare the safety profiles of each regimen (1, 23-25).
Previous research suggests that fentanyl can prolong analgesia but may increase the risk of respiratory depression (19, 20), while epinephrine may help mitigate hypotension through its vasoconstrictive effects (17). This investigation sought to refine current spinal anesthesia practices by providing evidence-based insight into the differential effects of these adjuvants, with the broader goal of improving patient safety, comfort, and perioperative outcomes across diverse surgical settings.
3. Methods
This quasi-experimental study was conducted between November 1, 2024, and January 31, 2025, at Imam Khomeini Hospital in Tehran, Iran. A total of 100 patients undergoing elective femur or tibia surgery under spinal anesthesia were enrolled. Ethical approval was obtained from the Ethics Committee of Tehran University of Medical Sciences (IR.TUMS.SPH.REC.1403.150).
3.1. Sample Size Calculation
The sample size was calculated using G*Power software based on the sensory block outcomes reported by Allen (26). Using the formula for two independent groups with a 95% confidence interval (α = 0.05, Z1 - α/2 = 1.96) and 80% power (β = 0.20, Z1 - β = 0.842), the required sample size was 31 participants per group. Sensory block means and standard deviations (m1 = 52.8, m2 = 39.6; S1 = 16.9, S2 = 20.6) yielded a pooled standard deviation of 18.84. To account for potential dropout, the final sample was increased to 50 patients per group.
3.2. Inclusion and Exclusion Criteria
Eligible participants were adult patients scheduled for elective femur or tibia surgery under spinal anesthesia. Inclusion criteria required patients to be classified as ASA physical status I - III and to provide informed written consent. Patients with controlled chronic conditions such as hypertension or diabetes were eligible, provided they were clinically stable. Blinding was maintained by ensuring that patients, data collectors, and the clinicians administering postoperative assessments were unaware of the group assignments. The anesthesiologist preparing the anesthetic solutions was not involved in patient care or data analysis, thus preserving allocation concealment. Exclusion criteria included known contraindications to spinal anesthesia, a history of opioid dependence or chronic pain requiring ongoing opioid therapy, and the presence of unstable or high-risk cardiopulmonary comorbidities. Patients unwilling or unable to participate, or those with incomplete data, were also excluded.
3.3. Randomization and Blinding
Participants were randomly allocated into two groups: One received bupivacaine with epinephrine, and the other received bupivacaine with fentanyl. All patients provided written informed consent after being fully briefed on the procedure, potential risks, benefits, and their right to withdraw at any time.
3.4. Preoperative Preparation
Preoperative assessments included a thorough medical history, medication review, physical examination, and confirmation of fasting status. Intravenous access was established, and standard anesthetic monitoring was applied. All spinal blocks were performed in the lateral decubitus position with the spine flexed, under sterile conditions.
3.5. Technique
All procedures were performed by a qualified anesthesiologist at the appropriate interspace using a standard spinal needle. In both groups, a 0.5% solution of bupivacaine was administered intrathecally. In the epinephrine group, 100 micrograms of epinephrine were added; in the fentanyl group, 35 micrograms of fentanyl were used. The total volume of the intrathecal injection was standardized at 3.5 mL in both groups.
3.6. Post-procedural Monitoring
After the spinal injection, patients were monitored for adequate sensory and motor block prior to transfer to the operating room. Vital signs were continuously observed, and supplemental oxygen was administered to maintain oxygen saturation above 94%. Intravenous fluids were given as needed to support hemodynamic stability. Signs of complications — including hypotension, bradycardia, respiratory depression, or allergic reactions — were closely monitored. Any protocol deviations were documented along with justifications.
3.7. Data Collection and Validation
Data were recorded using standardized case report forms. To ensure accuracy, a second investigator independently reviewed a random sample of forms. Acceptable ranges were predefined for key variables (e.g., blood pressure, oxygen saturation), and any outliers were investigated.
3.8. Statistical Analysis
Data were analyzed using SPSS software, version 25 (IBM Corp., Armonk, NY). Continuous variables were compared using the independent samples t-test, and categorical variables were analyzed using the chi-square test. A P-value of less than 0.05 was considered statistically significant.
3.9. Use of Artificial Intelligence Tools
The AI tools were used to support grammar checking, language refinement, and clarity improvement during the preparation of this manuscript. All content, analysis, and interpretations reflect the authors’ original work and scientific judgment.
4. Results
Table 1 summarizes the demographic and baseline characteristics of the study population. The mean age was 43.40 ± 17.97 years in the epinephrine group and 40.14 ± 15.27 years in the fentanyl group. Male participants comprised 60% of the epinephrine group and 64% of the fentanyl group. Regarding marital status, 62% of the epinephrine group and 72% of the fentanyl group were married. The mean number of children per patient was 1.66 ± 1.69 in the epinephrine group and 1.86 ± 1.40 in the fentanyl group.
| Patient Characteristics | Bupivacaine + Epinephrine (N = 50) | Bupivacaine + Fentanyl (N = 50) |
|---|---|---|
| Age | 43.40 ± 17.97 | 40.14 ± 15.27 |
| Gender (M/F) | 30/20 (60/20) | 32/18 (64/36) |
| Marital state (yes/no) | 31/19 (62/38) | 36/14 (72/28) |
| Child number | 1.66 ±1.69 | 1.86 ± 1.40 |
Parameters and Socio-demographic Finding in the Results of Two Groups a
As shown in Table 2, statistically significant differences were observed between the two groups with respect to muscle relaxation levels (P < 0.001). The incidence of hypotension was significantly higher in the bupivacaine plus epinephrine group (P < 0.001), as was the occurrence of bradycardia (P < 0.001). Conversely, pruritus was reported exclusively in the bupivacaine-fentanyl group, also reaching statistical significance (P = 0.001). Additionally, the proportion of patients who experienced no complications differed significantly between the two groups (P = 0.018), indicating meaningful differences in the overall safety profile.
| Variables | Bupivacaine + Epinephrine (N = 50) | Bupivacaine + Fentanyl (N = 50) | P-Value |
|---|---|---|---|
| Muscle relaxant level (MR) | |||
| No relaxation | 0 (0) | 0 (0) | - |
| Moderate relaxation | 8 (16) | 27 (54) | 0.000 |
| Complete relaxation | 42 (84) | 23 (46) | 0.000 |
| Complication incidence | |||
| No complication | 36 (72) | 42 (84) | 0.018 |
| Hypotension | 8 (16) | 0 (0) | 0.000 |
| Nausea | 1 (2) | 1 (2) | 1.000 |
| Bradycardia | 5 (10) | 0 (0) | 0.000 |
| Itching | 0 (0) | 7 (14) | 0.001 |
The Results of Some Related Factor of the Two Groups a
As shown in Table 3, the hemodynamic comparison between the bupivacaine-epinephrine and bupivacaine-fentanyl groups revealed several statistically significant differences across various time points. In the early postoperative period (5 to 15 minutes), peripheral oxygen saturation (SpO2) was significantly higher in the epinephrine group at 5 minutes (97.54% ± 1.65) compared to the fentanyl group (96.52% ± 1.26; P = 0.001), although no meaningful differences were noted at subsequent time points. In contrast, diastolic blood pressure (DBP) was consistently higher in the fentanyl group, with significant differences observed at both 10 minutes (79.38 ± 4.49 vs. 74.16 ± 9.47; P = 0.001) and 15 minutes (77.72 ± 5.09 vs. 73.36 ± 9.94; P = 0.007).
| Variables | Bupivacaine + Epinephrine (N = 50) | Bupivacaine + Fentanyl (N = 50) | P-Value |
|---|---|---|---|
| After 5 min | |||
| SBP | 117.16 ± 8.16 | 117.40 ± 4.67 | 0.857 |
| DBP | 73.96 ± 10.56 | 77.16 ± 4.94 | 0.055 |
| HR | 76.88 ± 12.67 | 74.90 ± 8.78 | 0.366 |
| SPO2 | 97.54 ± 1.65 | 96.52 ± 1.26 | 0.001 |
| After 10 min | |||
| SBP | 115.78 ± 5.21 | 117.84 ± 4.67 | 0.129 |
| DBP | 74.16 ± 9.47 | 79.38 ± 4.49 | 0.001 |
| HR | 74.06 ± 13.09 | 76.70 ± 9.18 | 0.246 |
| SPO2 | 97.08 ± 1.92 | 97.20 ± 1.30 | 0.716 |
| After 15 min | |||
| SBP | 116.20 ± 9.95 | 116.82 ± 4.53 | 0.680 |
| DBP | 73.36 ± 9.94 | 77.72 ± 5.09 | 0.007 |
| HR | 75.16 ± 12.08 | 75.04 ± 8.52 | 0.954 |
| SPO2 | 96.98 ± 1.84 | 97.48 ± 1.26 | 0.117 |
| After 20 min | |||
| SBP | 114.74 ± 8.97 | 118.70 ± 4.66 | 0.007 |
| DBP | 73.52 ± 10.09 | 77.72 ± 5.09 | 0.010 |
| HR | 73.74 ± 10.96 | 76.22 ± 8.62 | 0.212 |
| SPO2 | 97.46 ± 1.52 | 97.62 ± 1.27 | 0.571 |
| After 30 min | |||
| SBP | 114.84 ± 8.72 | 117.52 ± 4.94 | 0.062 |
| DBP | 72.40 ± 9.55 | 77.16 ± 5.06 | 0.002 |
| HR | 73.48 ± 10.37 | 75.42 ± 8.70 | 0.314 |
| SPO2 | 97.10 ± 1.50 | 97.28 ± 1.61 | 0.565 |
| After 1 h | |||
| SBP | 112.36 ± 16.57 | 116.60 ± 4.67 | 0.085 |
| DBP | 74.02 ± 8.95 | 77.34 ± 5.21 | 0.026 |
| HR | 72.22 ± 8.90 | 77.22 ± 8.10 | 0.004 |
| SPO2 | 97.44 ± 1.52 | 97.44 ± 1.41 | 1.000 |
| After 2 h | |||
| SBP | 115.86 ± 9.81 | 117.74 ± 6.06 | 0.252 |
| DBP | 75.56 ± 8.65 | 79.46 ± 14.71 | 0.109 |
| HR | 73.28 ± 9.24 | 75.02 ± 13.70 | 0.459 |
| SPO2 | 97.64 ± 1.52 | 94.92 ± 13.58 | 0.163 |
The Results of the Hemodynamic Status After the Procedure a
During the mid-phase (20 to 30 minutes), systolic blood pressure (SBP) was significantly higher in the fentanyl group at 20 minutes (118.70 ± 4.66 vs. 114.74 ± 8.97; P = 0.007). The DBP also remained significantly elevated in the fentanyl group at 20 minutes (77.72 ± 5.09 vs. 73.52 ± 10.09; P = 0.010) and 30 minutes (77.16 ± 5.06 vs. 72.40 ± 9.55; P = 0.002).
In the later phase (1 to 2 hours post-injection), HR was significantly higher in the fentanyl group at 1 hour (77.22 ± 8.10 vs. 72.22 ± 8.90; P = 0.004), with DBP also remaining elevated at that time point (77.34 ± 5.21 vs. 74.02 ± 8.95; P = 0.026).
Other parameters, including SBP (except at 20 minutes), HR (except at 1 hour), and SpO2 (except at 5 minutes), did not differ significantly between the groups at the remaining measured intervals. Overall, the bupivacaine-fentanyl group demonstrated a trend toward higher diastolic pressures throughout the 10 to 60-minute period, along with transient elevations in SBP and HR. Conversely, the bupivacaine-epinephrine group was associated with higher early oxygen saturation and more stable HR measurements in the initial 5 to 30 minutes. These findings suggest that each combination produces a distinct hemodynamic profile: Fentanyl contributes to more pronounced pressor effects, while epinephrine appears to offer better early oxygenation and HR stability.
Table 4 presents the comparison of anesthesia and surgery durations between the bupivacaine plus epinephrine and bupivacaine plus fentanyl groups. The mean surgery duration was 162.50 ± 30.27 minutes in the epinephrine group and 135.52 ± 19.69 minutes in the fentanyl group. Although the epinephrine group had a longer surgical time on average, the difference was not statistically significant (P = 0.122). In contrast, the duration of anesthesia differed significantly between the two groups. Patients receiving bupivacaine with epinephrine had a mean anesthesia duration of 222.38 ± 17.48 minutes, compared to 169.88 ± 15.57 minutes in the fentanyl group (P = 0.001), indicating that epinephrine significantly prolonged the duration of spinal anesthesia.
| Variables | Bupivacaine + Epinephrine (N = 50) | Bupivacaine + Fentanyl (N = 50) | P-Value |
|---|---|---|---|
| Surgery duration | 162.50 ± 30.27 | 135.52 ± 19.69 | 0.122 |
| Anesthesia duration | 222.38 ± 17.48 | 169.88 ± 15.57 | 0.001 |
Anesthesia and Surgery-related Variables Between the Groups a
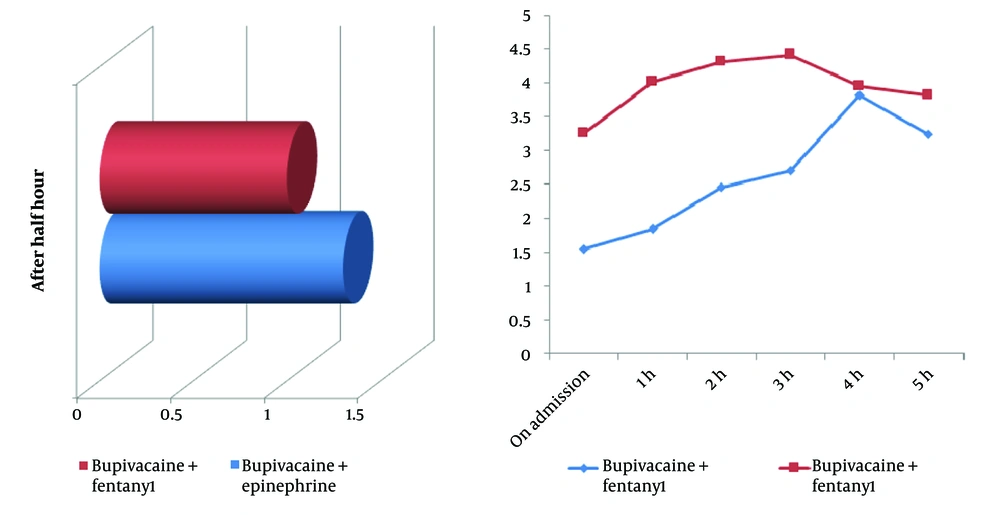
Postoperative pain scores differed significantly between groups at all time points, with the fentanyl group showing consistently lower scores for up to five hours postoperatively. In the early recovery phase, 30 minutes after surgery, the bupivacaine-fentanyl group reported significantly lower pain scores (1.0 ± 0.0) compared to the bupivacaine-epinephrine group (1.3 ± 0.73; P = 0.005), indicating more effective immediate analgesia. Upon admission to the ward, this trend persisted, with pain scores remaining lower in the fentanyl group (1.54 ± 0.57 vs. 3.26 ± 1.60; P < 0.001) (Figure 1A).
A, comparison of postoperative pain scores between groups after 30 minutes; this 3D bar chart illustrates the mean pain scores reported by patients 30 minutes after surgery in the two study groups: Patients who received bupivacaine plus fentanyl reported lower pain levels compared to those who received bupivacaine plus epinephrine; B, postoperative pain score trends over 5 hours in two anesthetic groups; this line graph shows the progression of pain scores from admission through 5 hours postoperatively for patients receiving spinal anesthesia. Patients in the bupivacaine plus fentanyl group exhibited consistently lower pain scores during the first 3 hours, while those in the bupivacaine plus epinephrine group experienced higher and more sustained pain levels over time.
The analgesic advantage of fentanyl continued throughout the early postoperative period. At 1 hour, 2 hours, and 3 hours post-admission, pain scores in the fentanyl group were consistently and significantly lower: 1.84 ± 0.54 vs. 4.02 ± 1.45 (P < 0.001), 2.46 ± 0.46 vs. 4.32 ± 1.24 (P = 0.003), and 2.70 ± 0.83 vs. 4.42 ± 1.03 (P < 0.001), respectively. Although scores between the groups began to converge during the late postoperative phase, the fentanyl group maintained a statistically significant advantage at 4 hours (3.82 ± 1.26 vs. 3.96 ± 1.06; P = 0.023) and 5 hours (3.24 ± 1.06 vs. 3.82 ± 1.10; P = 0.009) (Figure 1B).
Overall, the bupivacaine-fentanyl group demonstrated superior analgesia throughout the first five postoperative hours, with the most pronounced difference observed at 3 hours (mean difference: 1.72 points). These results suggest that intrathecal fentanyl offers more effective and sustained pain control than epinephrine, particularly during the critical early recovery phase, potentially contributing to greater patient comfort and earlier mobilization.
5. Discussion
This study compared the clinical effects of intrathecal bupivacaine combined with either epinephrine or fentanyl in patients undergoing femoral and tibial surgeries under spinal anesthesia. Key outcomes evaluated included anesthesia duration, postoperative pain control, muscle relaxation, hemodynamic changes, and complication profiles. The results provide insight into how each adjuvant influences anesthetic quality and perioperative stability.
Anesthesia duration was significantly longer in the epinephrine group (222.38 ± 17.48 minutes) than in the fentanyl group (169.88 ± 15.57 minutes; P = 0.001), consistent with epinephrine’s vasoconstrictive effect, which slows systemic absorption of local anesthetics. This finding aligns with Katz et al. (27), who observed that intrathecal epinephrine at higher doses (up to 200 µg) significantly prolonged block duration and delayed regression to the T10 dermatome. Similarly, Hamzei et al. (8) reported enhanced paralysis and analgesia when epinephrine was added to spinal anesthetic solutions. However, studies such as Goodman et al. (28) found no such benefit in obstetric populations, highlighting the influence of surgical context, epinephrine dose (our study used 100 µg), and assessment methods. Overall, epinephrine may be more beneficial in procedures requiring extended motor and sensory blockade.
In contrast, patients in the fentanyl group experienced significantly lower pain scores, particularly during the first 3 - 4 postoperative hours. This observation is supported by studies from Farzi et al. (29) and Gajbhare and Kamble (30), which demonstrated superior early analgesia with intrathecal fentanyl compared to bupivacaine alone. While Katz et al. (27) did not observe intraoperative differences between morphine and epinephrine, this may reflect variation in opioid type or surgical procedure. The short-term analgesic effect of fentanyl, mediated by spinal opioid receptor activation, appears especially effective in the early recovery period but diminishes by 4 - 5 hours, often necessitating supplemental analgesia.
Muscle relaxation was significantly more pronounced in the epinephrine group, with 84% of patients achieving complete relaxation compared to 46% in the fentanyl group. Although data on this specific outcome in orthopedic populations are limited, the finding is likely attributable to the prolonged motor blockade associated with epinephrine. Ferrarezi et al. (31) previously reported extended motor block in cesarean patients receiving fentanyl, but did not directly assess relaxation quality. In orthopedic surgery, enhanced muscle flaccidity is clinically desirable and may improve surgical conditions.
Regarding adverse effects, the epinephrine group had a higher incidence of hypotension and bradycardia, while pruritus occurred exclusively in the fentanyl group. Although the epinephrine group showed statistically significant increases in hypotension and bradycardia, the small differences in SBP and DBP (3 - 5 mmHg) are unlikely to have meaningful clinical consequences in ASA I–III patients undergoing elective surgery. These trends are consistent with earlier findings: Farzi et al. (29), Ferrarezi et al. (31), and Okutomi et al. (32) have all reported pruritus as a common side effect of intrathecal fentanyl, likely due to central opioid receptor activation. The hypotension observed in the epinephrine group aligns with concerns raised by Hamzei et al. (8), although we did not observe an increased rate of nausea, as reported by Goodman et al. (28). This discrepancy may be attributable to differences in patient population and perioperative management strategies.
Hemodynamically, both groups remained relatively stable, though the fentanyl group exhibited slightly higher diastolic pressures and HRs at multiple time points. Rambhia et al. (33) reported increased vasopressor requirements with higher doses of intrathecal epinephrine; in contrast, the low dose used in our study (100 µg) combined with proactive fluid management likely mitigated these effects. Some prior studies have reported no significant hemodynamic differences between adjuvants, suggesting that both can be used safely when dosing is carefully controlled and appropriate monitoring is in place.
While the observed hemodynamic differences (e.g., DBP 3 - 5 mmHg higher in the fentanyl group) were statistically significant, their clinical relevance warrants caution. Transient blood pressure variations of this magnitude are unlikely to necessitate intervention in ASA I - III patients but may merit closer monitoring in high-risk populations. Notably, the fentanyl group’s stable hemodynamic profile aligns with its superior safety outcomes, reinforcing its utility in patients prone to cardiovascular instability.
Taken together, our findings emphasize the importance of individualized adjuvant selection based on surgical duration, patient comorbidities, and perioperative goals. While epinephrine may be preferable for longer procedures requiring sustained motor block, fentanyl offers superior early postoperative analgesia with fewer hemodynamic complications, making it a suitable choice for shorter surgeries or in patients at higher cardiovascular risk.
In summary, our findings partially align with existing literature, reinforcing the known pharmacologic actions of epinephrine, which prolongs block duration through vasoconstriction, and fentanyl, which provides potent early analgesia via opioid receptor activation. Differences observed across studies may be attributed to variations in adjuvant dosing, surgical type, patient demographics, and outcome assessment techniques.
From a clinical standpoint, bupivacaine combined with epinephrine appears more suitable for longer orthopedic procedures requiring sustained motor block, whereas the fentanyl combination.
5.1. Conclusions
This study demonstrated that the combination of bupivacaine with epinephrine resulted in a significantly longer duration of anesthesia but was associated with a higher incidence of complications, including hypotension and bradycardia. In contrast, bupivacaine combined with fentanyl provided better postoperative pain control, greater hemodynamic stability, and fewer adverse effects overall. Based on these findings, bupivacaine plus fentanyl may be the preferred choice for spinal anesthesia in lower limb surgeries where early postoperative comfort and safety are priorities. However, in cases requiring prolonged anesthesia or deeper motor block, bupivacaine with epinephrine may still be advantageous. Selection of the optimal adjuvant should be guided by the clinical context, patient comorbidities, and surgical duration.offers a better option for shorter surgeries where early postoperative comfort and rapid recovery are prioritized.
5.2. Limitations
As a quasi-experimental, single-center trial without full randomization or blinding, the potential for selection bias and limited internal validity exists. The relatively small sample size and surgical population may limit generalizability to other surgical populations. We emphasize the need for future randomized controlled trials with larger and more diverse populations.