1. Background
Diabetes mellitus is one of the increasing socio-medical problems (1). This metabolic disease involves the brain as well as beta cells of pancreas, muscles, and liver (2). There are several reports indicating that diabetes mellitus has a devastating effect on central nervous system, including a decrease in electrical connection between brain structures, impaired memory and learning, decrease in axonal transport, and difficulties in problem solving (3-8). Our previous study showed that the projections of raphe nuclei to hippocampal CA3 region in diabetic rats were less than nondiabetic group (9). Peripheral neuropathy, development and progression of depressive illness, and neural death are late complications of diabetes (10, 11). Mesencephalic raphe nuclei (dorsal and median) are the main serotonin resources innervating the forebrain (12-14). The median raphe nucleus (MnR) exists rostral to the raphe pontis nucleus and consists of rostral and caudal subdivisions (9). Dorsal raphe (DR) nucleus is the largest serotonergic nucleus located in the ventral periaqueductal grey mater (PAG) which is divided into rostral and caudal parts (9, 15). Neostriatum or caudoputamen is somatotopically organized and involved in planning and modulation movement pathways (13, 16). This area is innervated by projections of raphe nuclei (17). Hyperglycemia results in increased blood flow in striatum and thalamus and causes hemichorea due to hyperactivity of neurons (18). Furthermore it impairs pro-oxidant/antioxidant balance and increases production of reactive oxygen species and lipid peroxidation in CNS (19). Therefore diabetes may affect survival and connections of neurons, and study different parts of the brain following induction of diabetes can be helpful in understanding the brain function.
2. Objectives
Because a decrease in the projections can cause a reduction in the neuron connections and neural messaging defects, this study was designed. The main objective of this study was to determine the effects of diabetes on projections of mesencephalic raphe nuclei to striatum of rats.
3. Materials and Methods
3.1. Animals
48 male adult Wistar rats of 210 - 230 g body weight were divided into four groups (12 in each group) in simple randomized manner. They were housed under controlled temperature (24ºC) and lighting conditions (12 hours light/dark cycle). Food and water were available ad libitum. All animal care and procedures were in accordance with the ethics of ethical committee of Tehran University of Medical Sciences.
3.2. Induction of Diabetes
Diabetes was induced by single intraperitoneal injection of 60 mg/kg Streptozocin (STZ; Sigma. St Louis Mo, The USA; dissolved in 0.9% sterile Saline). Rats were divided into the following groups: 1) control, 2) two month diabetic, 3) four month diabetic, and 4) six month diabetic. Hyperglycemia was confirmed using a strip-operated blood glucose sensor in a blood sample obtained by tail pick six days after the STZ injection.
3.3. Track Tracing
The animals were anesthetized by intraperitoneal injection of Ketamine (50 mg/kg) and Xylazine (10 mg/kg) and were given single stereotoxic injection of 0.5 µL HRP (Sigma, type VI) into the dorsal and ventral striatum separately as described in Paxinos Atlas. After 48 to 72 hours, which is the survival time the animals were killed with Ketamine and Xylazine, and perfused intracardiacly with 100 mL normal saline, 500 mL fixative solution (Glutaraldehyde 1.25% and Paraformaldehyde 1% in 0.2 M phosphate buffer at pH = 7.4), and sucrose buffer 10%, respectively. The brains were removed and post-fixed overnight (4ºC). 40 µm frozen coronal sections were taken and reacted with tetra methyl benzidine (Sigma. St Louis. Mo, The USA) to reveal HRP activity. Sections were mounted on gelatinized slides and counterstained with 1% neutral red.
3.4. Labeled Neurons Counting
Serial sections were provided from tissue in 40µm slices and in every 10, one slice was chosen for neuron counting. The extent of injection sites and distribution of retrogradely labeled neurons in dorsal and median raphe nuclei were counted by optika software.
3.5. Statistical Analyses
All data were expressed as mean values and their standard errors (S.E.). SPSS 16 software was chosen for analysis of the data. For analyzing the four groups of study one way-ANOVA test and Post Hoc of Tukey were chosen. The statistical significance level was set at P ≤ 0.05.
4. Results
4.1. Blood Sugar and Body Weight
The control animals showed a normal increase in body weight from 210 ± 20 to 410 ± 5 g; whereas, body weight decreased in the STZ-diabetic group from 210 ± 20 to 130 ± 20 g. In control group there was not any change in blood glucose level (80 - 110 mg/dL), but in diabetic group blood glucose levels increased to > 300 mg/dL.
4.2. Projections of Dorsal Raphe and Median Raphe Nucleus Nuclei to Dorsal Striatum
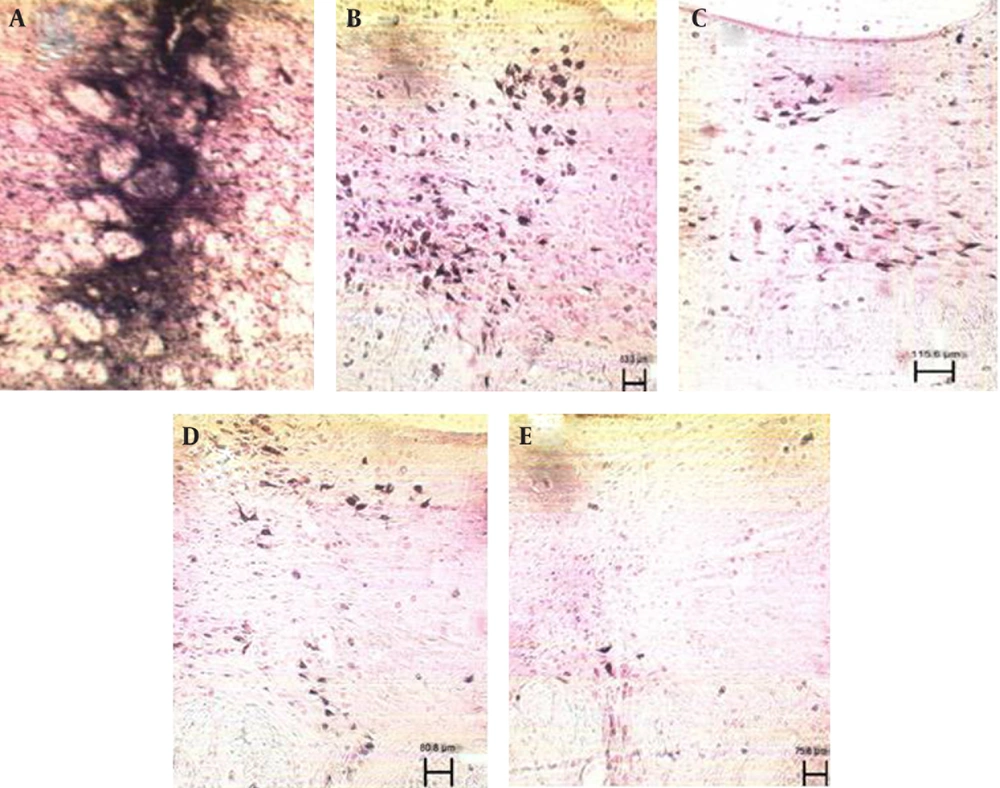
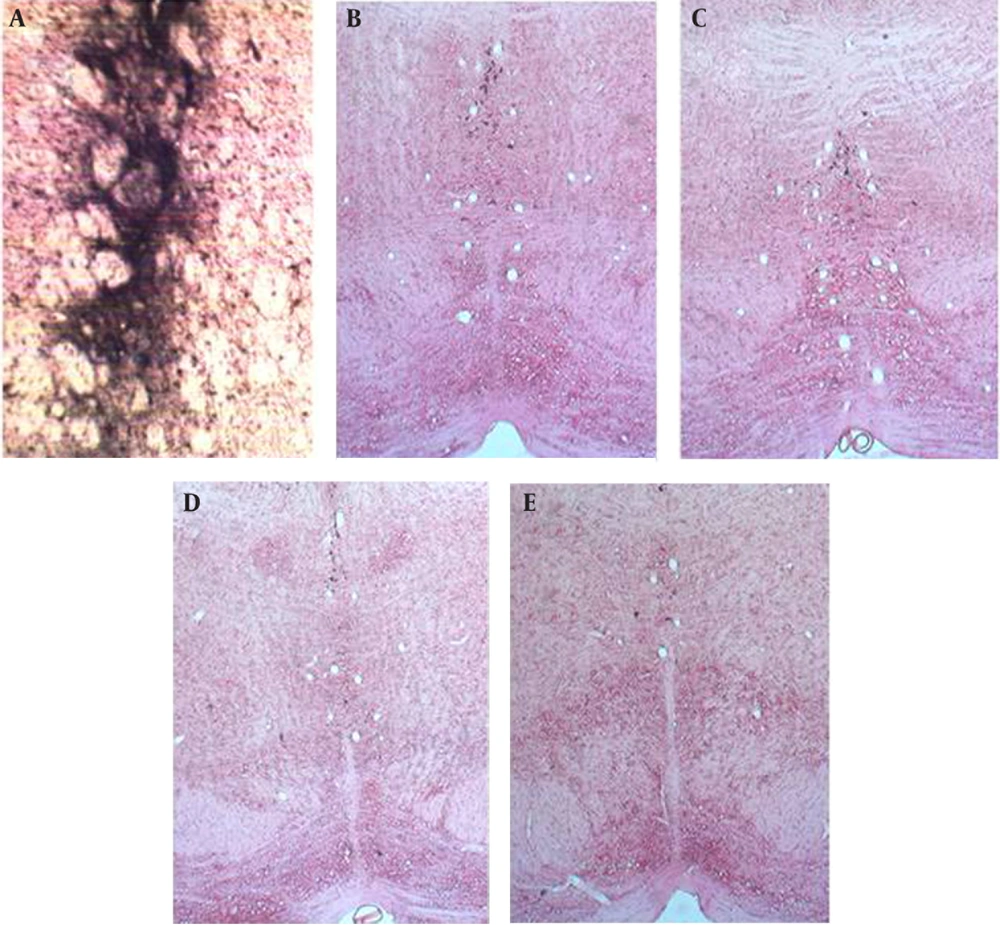
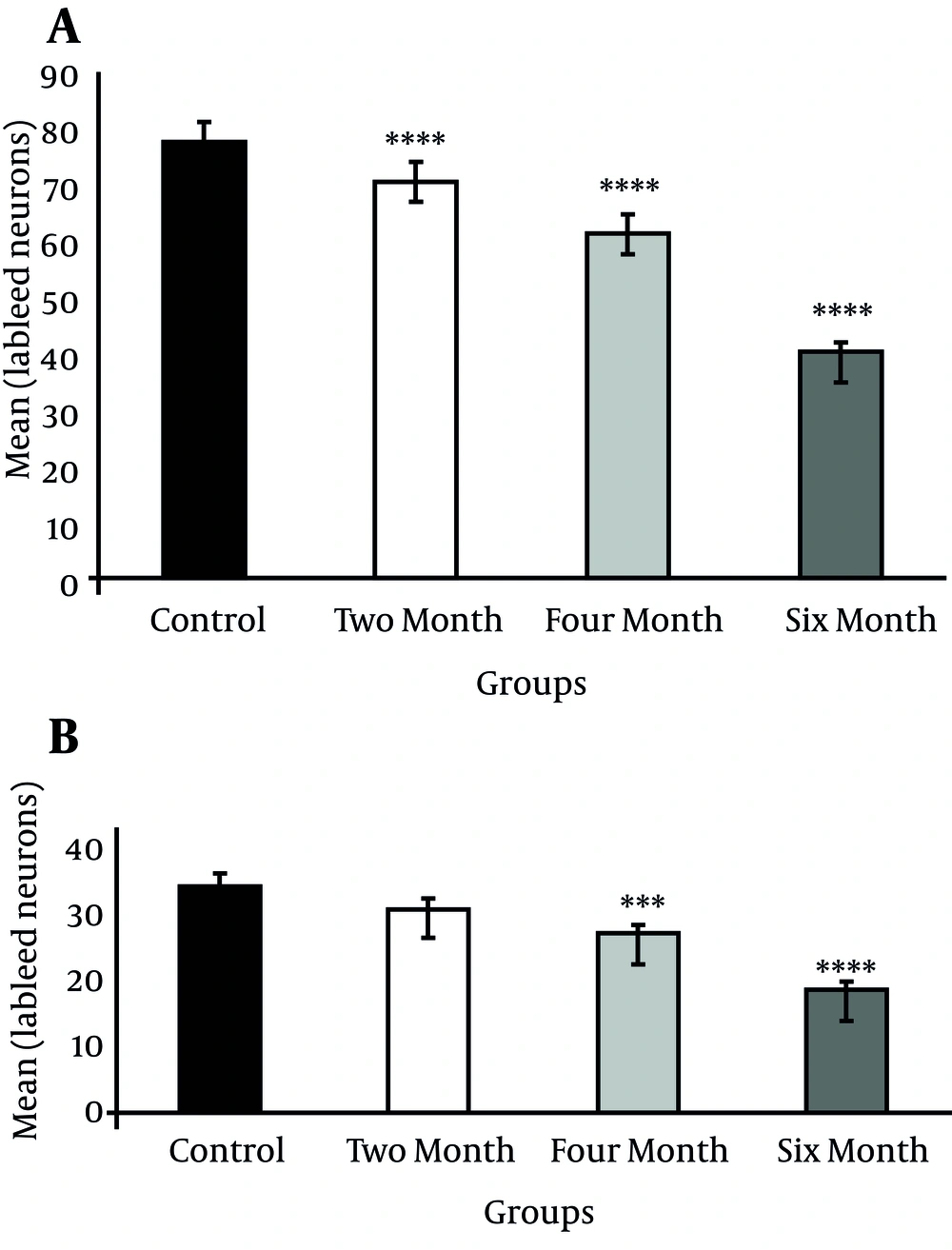
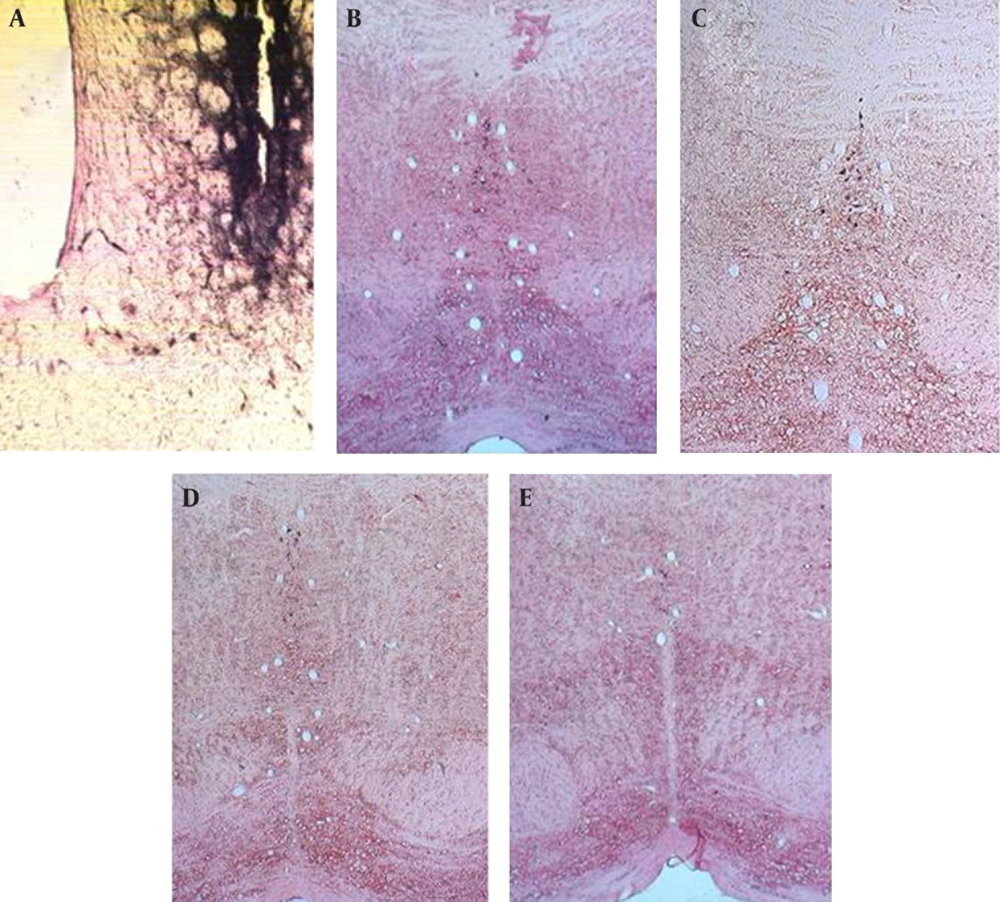
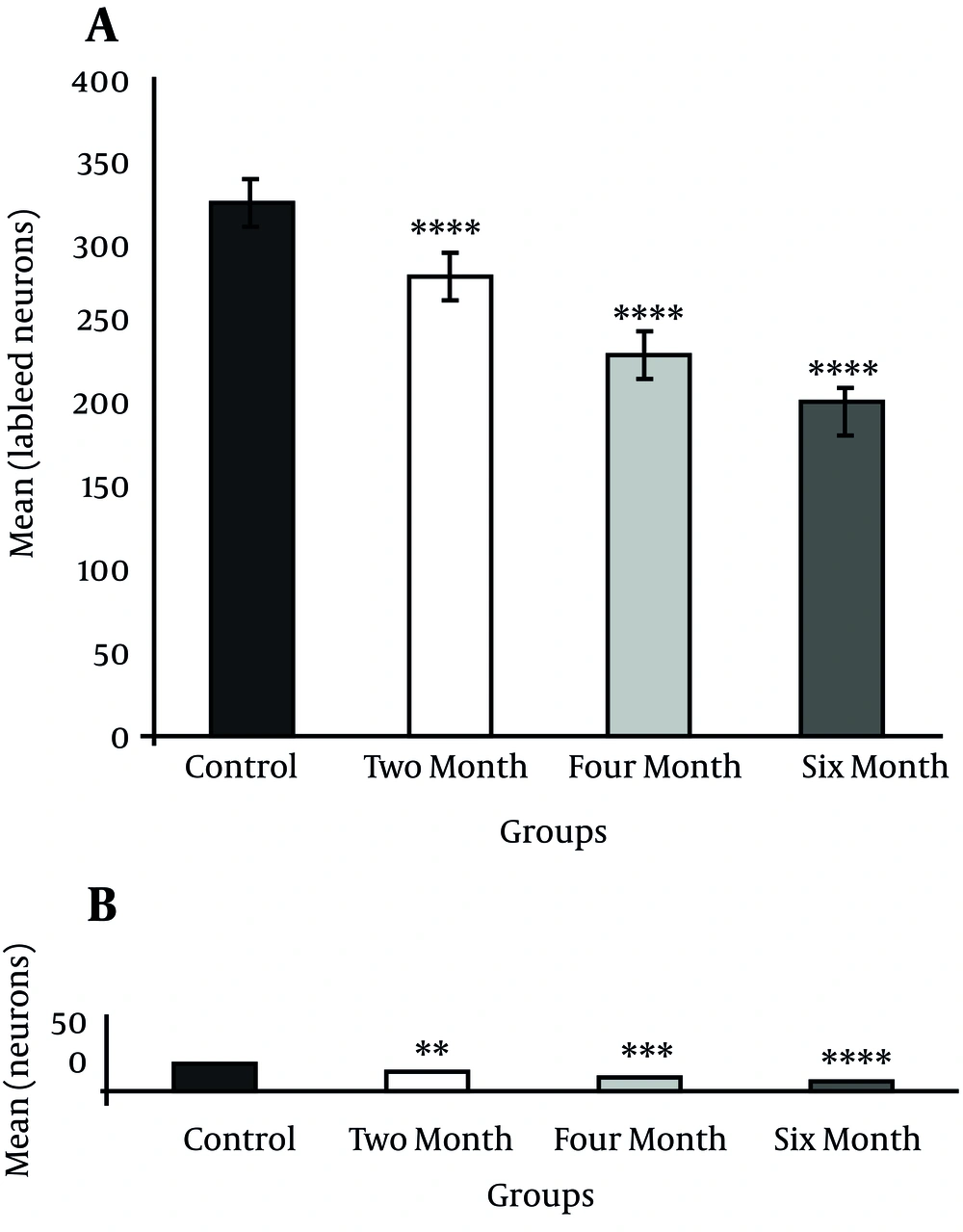
Injection of HRP into the dorsal striatum indicated a reduction in labeled neurons of the DR and MnR nuclei in all diabetic groups (Figure1 and 2). Reduced number of labeled neurons was significant in DR nucleus (P ≤ 0.0001); whereas, no significant reduction was observed in MnR nucleus two months after the induction of diabetes. Decrease of labeled neurons in MnR became significant in the four and six month groups after injection of STZ respectively with P ≤ 0.001 and P ≤ 0.0001 (Figure 3).
A) Photomicrograph of dorsal striatum after Injection of HRP, B) Labeled neurons in dorsal raphe nucleus of Control group, scale bar 83.3µm, C) Labeled neurons in dorsal raphe nucleus in two month diabetic group, scale bar 115.6µm, D) Labeled neurons in dorsal raphe nucleus in four month diabetic group, scale bar 80.8µm and E) Labeled neurons in dorsal raphe nucleus in six month diabetic group, scale bar 75.8µm (X 100).
A) Photomicrograph of dorsal striatum after Injection of HRP, B) Labeled neurons in median raphe nucleus of Control group, C) Labeled neurons in median raphe nucleus in two month diabetic group, D) Labeled neurons in median raphe nucleus in four month diabetic group, and E) Labeled neurons in median raphe nucleus in six month diabetic group, scale bar 75.8µm (X 100).
4.3. Projections of Dorsal and Median Raphe Nuclei to Ventral Striatum
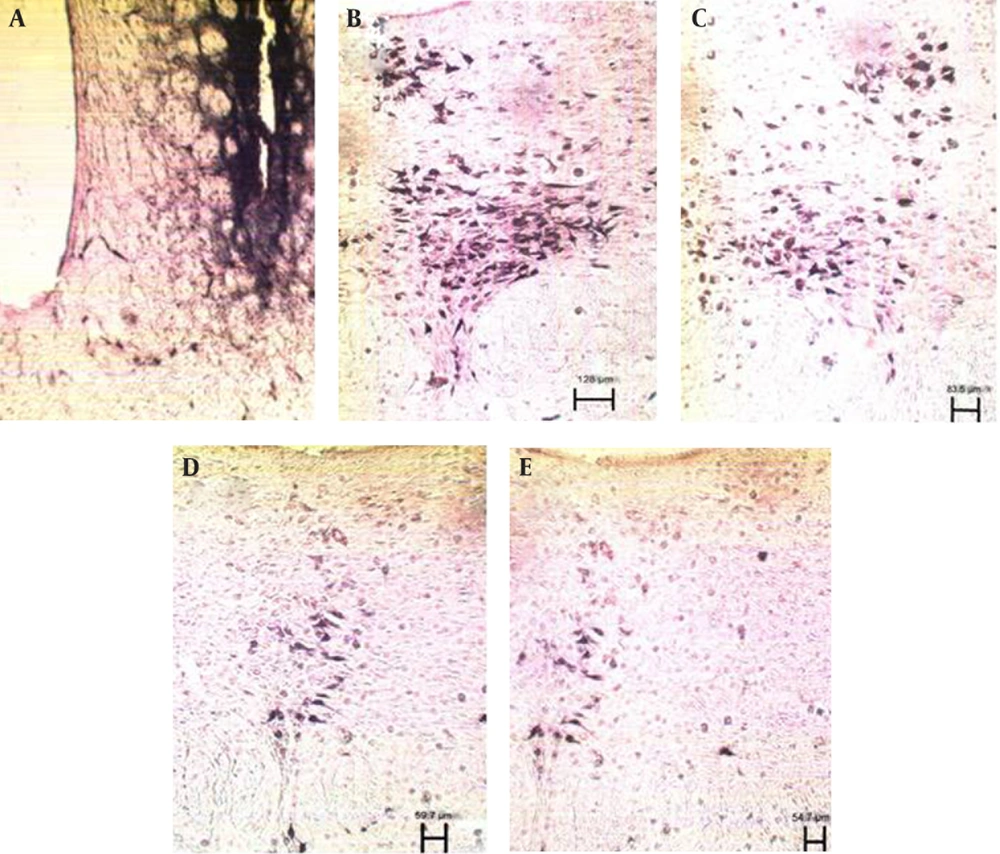
After injection of HRP into the ventral striatum, labeled neurons of DR and MnR nuclei decreased significantly, but this reduction was more significant in DR (P ≤ 0.0001) in comparison to MnR (P ≤ 0.01) two months after injection of STZ (Figure 4 and 5). Two months later, the number of decreased neurons was significant in both nuclei (P ≤ 0.0001) (Figure 6).
A) photomicrograph of ventral striatum after Injection of HRP, B) Labeled neurons in dorsal raphe nucleus of Control group, scale bar 128µm, C) Labeled neurons in dorsal raphe nucleus in two month diabetic group, scale bar 83.5µm, D) Labeled neurons in dorsal raphe nucleus in four month diabetic group, scale bar 59.7µm, and E) Labeled neurons in dorsal raphe nucleus in six month diabetic group, scale bar 54.7µm (X 100).
A) photomicrograph of ventral striatum after Injection of HRP, B) Labeled neurons in median raphe nucleus of Control group, C) Labeled neurons in median raphe nucleus in two month diabetic group, D) Labeled neurons in median raphe nucleus in four month diabetic group, and E) Labeled neurons in median raphe nucleus in six month diabetic group, scale bar 54.7µm (X 100).
5. Discussion
According to the results of this study, projections of DR nucleus to ventral striatum were more than of those to dorsal striatum in the control group. These findings are in accordance with previous studies (13-20). A significant decrease in DR efferents to dorsal and ventral striatum was found two months after the STZ injection. Our findings showed less labeled neurons in MnR nucleus in comparison to DR nucleus after injection of HRP into the dorsal and ventral striatum. This result is confirmed by our previous study (13). Two months after the STZ injection, MnR efferents to dorsal striatum were reduced, but it was not significant. However, this reduction became significant two months later. MnR projections to ventral striatum decreased significantly two months after diabetes induction, and this reduction remained significant after four months. The MnR projections to ventral striatum are affected by diabetes mellitus more than that of those to dorsal striatum. Study of Shapakov and colleagues showed that the level of serotonin in striatum is not significantly different between diabetic and nondiabetic rats (21). On the other hand, some studies indicate that induction of diabetes mellitus causes a decrease in serotonin level of the striatum (22, 23). We observed a decrease in projections but it is difficult to distinguish serotonergic neurons from non-serotonergics in our study. Tung and colleagues claimed that hyperglycemia can cause transient and reversible effects to basal nuclei functions (24). Furthermore, previous studies showed axonal transport reduction and cell death following diabetes mellitus (3). Our study indicated a decrease in projections of DR and MnR nuclei to striatum, which would not be transient or reversible if caused by cell death. Hence, more studies are required to clarify the exact relationship between diabetes mellitus and cell death in mesencephalic raphe nuclei (25, 26). Serotonergic neurons of DR mediate feeding behaviors. Ventral striatum can be activated by food stimuli because of its relation to limbic system (25, 26), and DR efferents to ventral striatum are more than of dorsal striatum. Therefore, diabetes-induced decrease in the number of efferents from DR to ventral striatum seems rational. The reduced number of projections from DR to dorsal striatum might influence the motor function (16), which needs to be confirmed by behavioral tests.
The numerous projections of DR and MnR nuclei to diverse regions of the brain suggested that these mesencephalic raphe nuclei are common targets of impairment in diabetes mellitus. We concluded that diabetes mellitus decreases the projections of mesencephalic raphe nuclei to the dorsal and ventral striatum.