1. Background
Stroke is one of the major causes of death in developed countries. About 85–90% of strokes result from cerebral infarction (1). Delayed cell death in CA1 pyramidal neurons of hippocampus has been induced by transient cerebral ischemia after 2 to 3 days of reperfusion (2, 3). The mechanisms underlying ischemic delayed neuronal death are unknown. Cysteine proteases of the interleukin-1β family (caspases), are activated by brain ischemia, which trigger neuronal apoptosis (4, 5). However, the mechanism that causes the activation of caspase after brain ischemia is not fully understood.
Behavioral and cognitive impairment are the most visible symptoms of transient global cerebral ischemia (TGCI). Spatial learning and memory deficits are two parameters that are most often described in rodent models of TGCI. Thus, the use of long-term functional/ behavioral end-points is an important requirement for evaluating the effect of drugs to treat ischemic brain damage (STAIR, 1999). It is necessary to find effective strategies and drugs for ameliorating or preventing brain ischemia/reperfusion (I/R) injury but only a few have been applied successfully in clinical practice, although several strategies and drugs have been shown to ameliorate brain ischemic damage in animal experimental models (6, 7).
It has been demonstrated that immunophilin ligands can be considered as a useful strategy in evaluation of neuroprotectors. It is observed that tacrolimus (FK506), a fungal-derived macrolide, has potent immunosuppressant effects which can provide neuro-protection against glutamate-induced neurotoxicity in animal models of focal (8-10) or transient, global forebrain ischemia (11, 12). Many studies have shown that FK506 reduces hippocampal pyramidal cell death after TGCI in the gerbil (11, 12) and rat two-vessel occlusion (2-VO) models (13). This effect is due to the interaction of FK506 with the immunophilin FKBP12 (FK506-binding protein) by inhibiting phosphatase calcineurin (14).
Calcineurin (CaN) is a Ca2+ and a calmodulin-dependent phosphatase, which plays an important role in neuronal response to Ca2+ concentration changes in the intracellular environment and in numerous physiological processes such as learning and memory. Decreasing CaN expression induces many pathological changes seen in brain ischemia (15). The neuro-protective effects of immune-suppressants are not well understood and many hypotheses have suggested that it may depend on new protein synthesis rather than inhibition of calcineurin (16, 17). Another concern about the therapeutic window of action is the time elapsed between the onset of ischemia and the beginning of treatment for which earlier use of drug would be more effective, and there have been a few studies about the time course of FK506. Also the relationship between structure and function is very important and we have shown the correlation between behavioral deficits measured in the Morris water maze and extend of pyramidal cell loss in the CA1 sector of the hippocampus.
2. Objectives
The present study was designed to investigate the neuro-protective efficacy and the time window for effective therapeutic use of FK506. FK506 delivery started immediately after beginning of reperfusion and before ischemia.
3. Materials and Methods
3.1. Animals and Drug Administration
Male Wistar rats weighing 250 to 300 g were housed in a controlled environment at 25 ± 2 °C with alternating 12-h light and dark cycles. All rats were acclimatized in our animal facility for at least 5 days prior to the experiments. Animal care was approved by the ethics committee, for the use of experimental animals at the Tehran University of medical sciences. An in situ cell death detection kit was purchased from Roche (Germany) and FK506 (solution, 5 mg/ml ampoule) was a gift from Astellas Pharmaceuticals (Osaka, Japan).
Animals were randomly allocated into four different groups as described below:
- Control group: rats were anesthetized by 40mg/kg of pentobarbital sodium, 30 min prior to ischemia (n = 8).
– I/R group (treatment 1): rats were subjected to the surgical procedure. (n = 8)
- FK506+ I/R group (treatment 2): rats were injected with FK506 1 mg/ kg, i.v, 20 minutes prior to I/R (n = 8).
- IR+ FK506 group (treatment 3): rats were injected with FK506, 1 mg/ kg, i.v, at the beginning of reperfusion (n = 8).
After the procedure, animals were kept in an animal house for one week, and then Morris water maze was performed. Rats were sacrificed 1 day after the end of the Morris water maze training.
3.2. Surgical Procedure
After anesthetization by sodium pentobarbital (40 mg/kg, IP), transient global ischemia was induced by occlusion of both common carotid arteries using Yashargil Aneurism micro clips for 20 minutes. During ischemia, body temperature was monitored and maintained at 37°C with a rectal probe and a heating lamp. Rats that had lost the righting reflex had dilated pupils and were unresponsive to gentle touch. Subsequently, the carotid arteries were released by removing the clips and restoration of blood flow was confirmed by observation of the carotid arteries. Animals were then returned to the animal house and kept for 1 week before performing the Morris water maze test. After intra-cardiac perfusion, rats were killed and their brains were removed, fixed in 4% paraformaldehyde and embedded in paraffin.
3.3. The Morris Water Maze (MWM)
Spatial learning and memory performance were measured using the MWM task. A circular plastic pool (height: 45 cm, diameter: 170 cm) was filled with water maintained at 22 ± 1°C and surrounded by a variety of extra-maze cues. The tank was divided into four quadrants and the escape platform (diameter: 18 cm) was located in the southeast (SE) quadrant which submerged 2 cm below the surface of the water at a fixed position. In the spatial acquisition phase, the rats learned to find a submerged platform using the extra-maze cues. Each rat participated in 16 trials, which were organized into daily blocks of 4 trials for 4 consecutive days. The rats were allowed to swim freely for a maximum of 60 seconds or until the platform was located. If the platform was not located during this time, the rat was guided to the platform and allowed to remain there for 20 second. Scape latencies (s), swim distance (cm), and swim speed (cm/s) were recorded. All rats were habituated to the water and apparatus 24 hours before water maze testing.
3.4. Histopathology
One day after ischemia, rats were anesthetized intraperitoneally with pentobarbital-Na (40 mg/kg) and transcardic perfusion was then performed with heparin (10 U/ml) in 0.9% saline, followed by 4% paraformaldehyde in 0.1 M phosphate buffer (Ph = 7.4). Their brains were removed and post –fixed in the same fixator for more than 3 days. Paraffin-embedded coronal sections were cut from Bregma (2/3 mm to 5 mm) into 3 and 10μm thick sections posterior to the bregma fortune for TUNEL and Nissl staining.
3.5. Nissl Staining
Continuous coronal sections (10 μm in thickness) of rat brain were prepared. These sections were processed with 1.0% cresyl violet, dehydrated, and cover slipped with Entellan. Histological changes in the brain were observed under an optical microscope. Image analysis of Nissl-stained slices was performed and numbered by using a light microscope. The CA1 pyramidal cells of the hippocampus in stained sections (3 sections of the hippocampus of each rat) were counted. Photomicrographs were taken at ×400 magnification with a microscope (Olympus AX-70) and analyzed by the image tool 2 software.
3.6. Tunel Staining
To detect neuronal apoptosis, Paraffin blocks were cut into 3 µm thickness coronal sections followed by TUNEL staining according to the protocol of the In Situ Cell Death Detection Kit (Roche, Mannheim, Germany). Briefly, for each rat, 10 coronal paraffinized sections were randomly selected and deparaffinized in xylene, hydrated by immersion in baths featuring an (decreasing) ethanol gradient, then washed in phosphate-buffered saline (PBS) and permeabilized by proteinase K (20 µg/ml) for 30 minutes at room temperature. Next, each section was incubated with 3% H2O2 in methanol for 10 minutes to block endogenous peroxidase (POD). Sections were incubated in the TUNEL reaction mixture at 37ºC for 60 min and rinsed with PBS then visualized by using converter-POD for 30 min at 37ºC and a humidified atmosphere. Sections were rinsed with PBS then 50-100 µl DAB substrate [diaminobenzidine (DAB)] was added and rinsed with PBS. All slides were mounted by a cover slip and analyzed by a light microscope at ×400 magnification. 10 randomly selected sections of each target area (CA1 area of hippocampus) were averaged to calculate the number of TUNEL-positive cells. All counting procedures were performed blindly.
3.7. Statistical Analysis
The results were presented as mean ± standard deviation (SD) observations. The significant difference was determined by a one-way ANOVA, followed by the Tukey's Multiple Comparison test. Statistical significance was defined as a P value ≤ 0.05.
4. Results
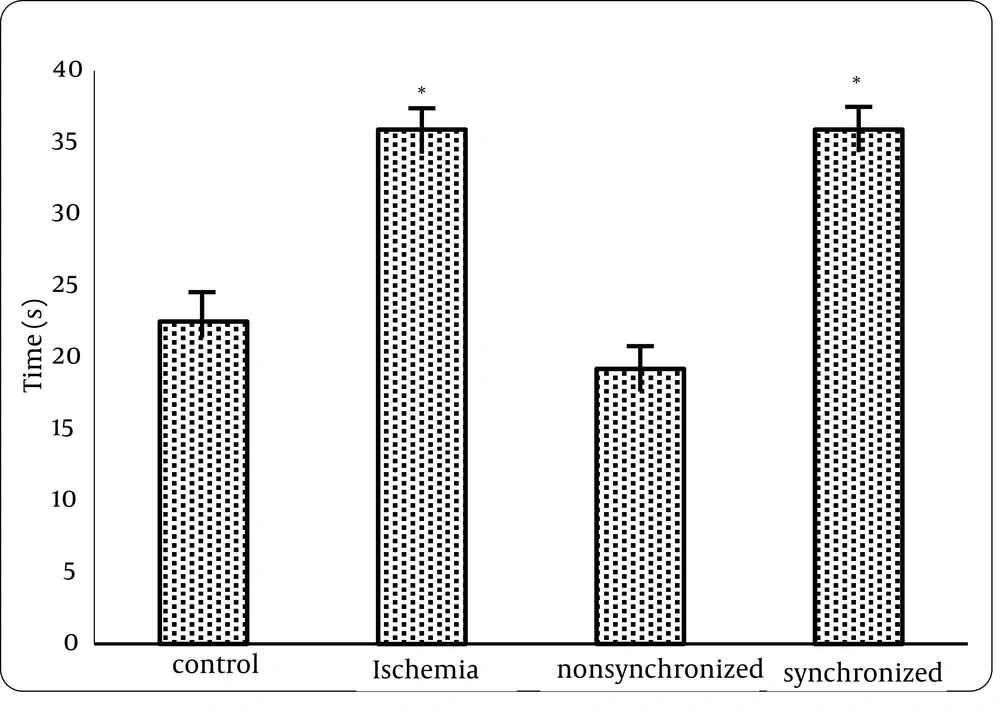
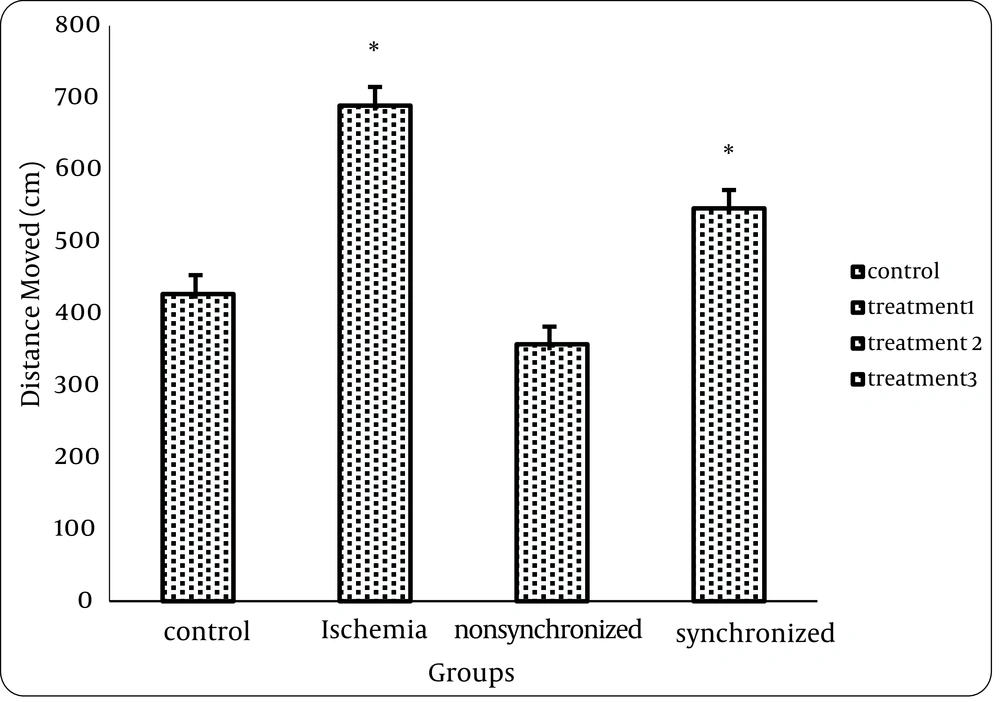
1) Analysis of data from the Morris water maze test showed that there was a statistically significant difference between the control and treatment1 (ischemia) group but no statistical differences were seen between control (intact) and treatment2 group neither in distance nor in time. This means that FK506 can improve spatial memory of animals, if injected 20 minutes before ischemia. (Figures 1 and 2)
2- Data from Nissl staining showed that 20 minutes of bilateral common carotid occlusion caused marked CA1 cell loss. But statistically significant differences were seen between treatment1 (ischemia) group and treatment 2 (P = 0.014) and treatment3 groups (P = 0.012). (Figure 3)
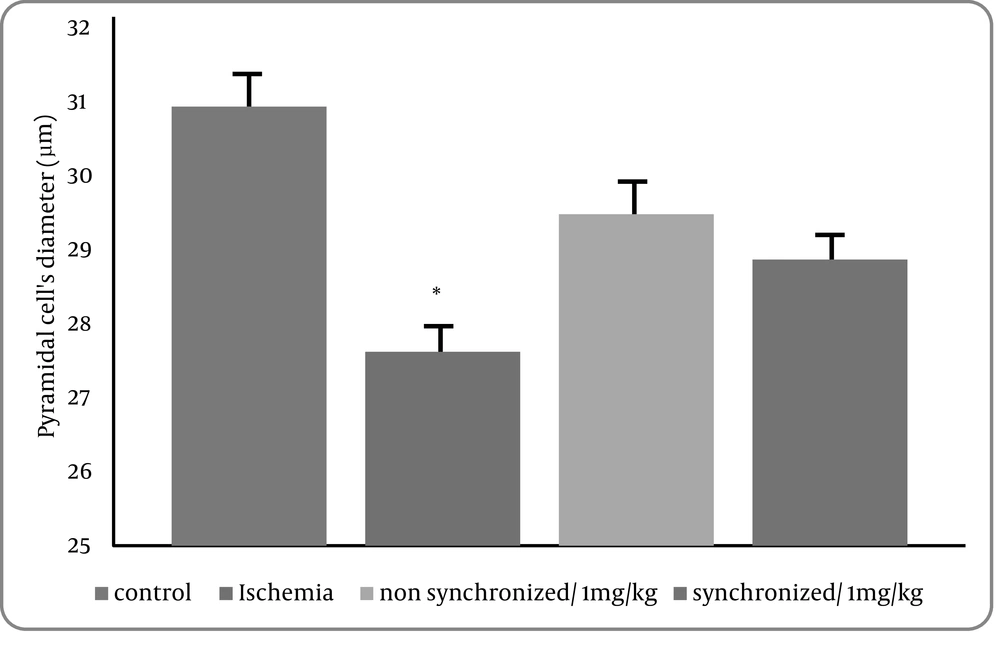
3) Data collected from cell diameters showed that 20 minutes of bilateral common carotid occlusion caused marked reduction of cell diameters in CA1 region of hippocampus. There was a statistically significant difference between the control versus the ischemia(treatment1) group (P = 0.03). However, there was no statistical significance between control group and treatment groups 2 and 3 (Figure 4).
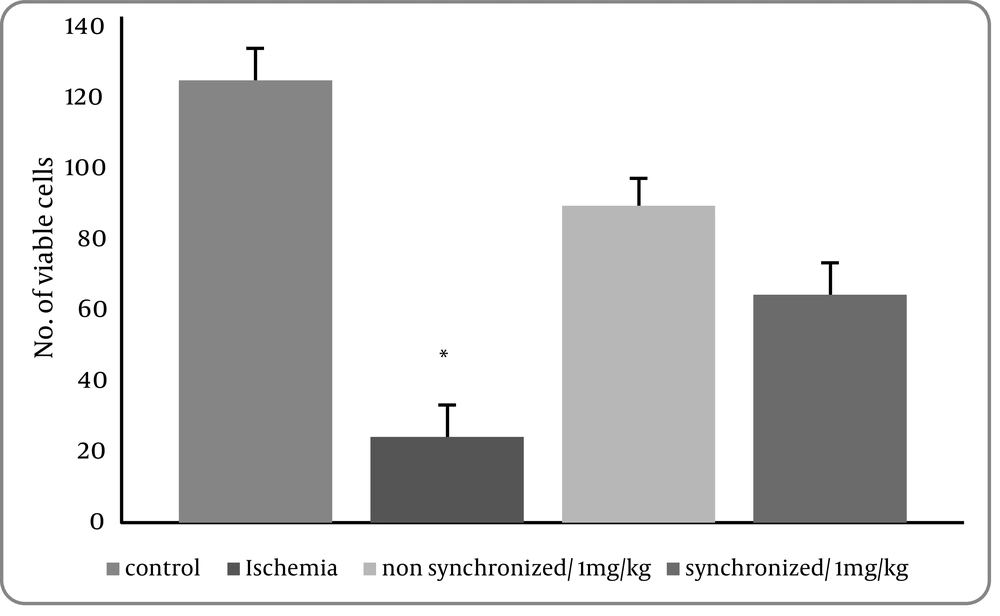
4) We next carried out TUNEL staining, which detects DNA damage characteristics of apoptosis after bilateral common carotid occlusion for 20 minutes. The number of TUNEL-positive cells was significantly increased compared with the control group in the CA1 region of hippocampus after ischemia. There was a significant deference between the control group versus the treatment1 (ischemia) group (P = 0.001) but no statistically difference between the control group and treatment groups 2 and 3 were seen. It means that apoptotic cells were significantly decreased in this region of the hippocampus by injection of FK506 before and after the ischemia. (Figure 5).
5. Discussion
CA1 neurons of the hippocampus are particularly sensitive and undergo selective and delayed degeneration in response to global cerebral ischemia (2, 3). These pyramidal neurons are critically involved in spatial learning and memory, and degeneration of these neurons results in learning and memory deficiencies (18). STAIR (1999) considers three major factors for evaluation of preclinical efficacy of neuro-protective drugs, including neuro-protection maintenance, time window and functional repairmen. According to these three important factors, we evaluated the efficacy of Tacrolimus in transient global brain ischemia treatment.
FK506 is known to reduce neuronal brain damage in experimental models of cerebral ischemia (19). Some research has shown that FK506 ameliorates the functional impairment caused by hypoxic (20) or focal ischemia (19) and following intracerebral hemorrhage (21) due to brain damage. The protective effect of FK506 also has been demonstrated after in vitro ischemia (22) and thrombotic ischemia stroke (23). However, there are no data about the effect of FK506 on functional recovery after transient global brain ischemia. The present this study has not only supported previous findings, but has also extended them from the histological to the functional level. The Stroke Therapy Academic Industry Roundtable (STAIR, 1999) emphasizes that behavioral measurements are very important in the preclinical evaluation of neuro-protective effects of drug before the beginning of clinical experiments. In the present study, FK506 significantly reduced the effect of ischemia on spatial and learning memory. Our data agrees with other studies showing that FK506 reduces the functional deficits associated with acute and cerebral ischemia (19). Our findings do not confirm Benetoli's who suggests that FK506 is not effective in treating the behavioral consequences of TGCI, but agrees with the second part of his research, which is concluded that FK506 is effective in reducing CA1 hippocampal damage (24).
Our data also confirms the hypothesis that FK506 may be an effective pharmacological strategy to treat the structural and functional outcomes of cerebral ischemia. The results of this study showed that FK506 can ameliorate histomorphologic changes of hippocampus and behavioral impairment due to brain ischemia/ reperfusion. According to pharmacotherapy of brain damages, the time of ischemia onset and the beginning of treatment is another important factor for evaluating the therapeutic action of drugs. Many studies have shown the time window of neuro-protective efficacy for FK506 in the rat model of cerebral ischemia. Butcher et al. (9). demonstrated that FK506 is effective when it has been administrated 2 to 3 h after focal brain ischemia, but some studies have been shown that this therapeutic time (1 h) for FK506 is shorter (10, 25). In this study, the neuroprotective effect of FK506 was maintained for at least 2 weeks, this finding agrees with previous studies showing that the neuro-protective effect of FK506 persists up to 45 (26) or 30 days after transient global ischemia (27).
In this study, a positive correlation between the behavioral impairment and pyramidal cell loss in CA1 region of the hippocampus has been detected. Our findings agree with the Bachevalier and Meunier study who reviewed the relationship between hippocampal cell loss and cognitive deficits due to brain ischemia (28), also Rod, Kiyota and Milani (29-31). However findings of Green and Nunn did not show any correlation between these two parameters (1, 32). This controversy may be due to the fact that the number of preserved, intact pyramidal cells may not be indicative of behavioral changes and other intra or extra hippocampal effects may define cognitive deficit by ischemia.
6. Conclusions
In conclusion, FK506 treatment before ischemia reduces cognitive impairments and neuronal death in the hippocampus induced by transient, global cerebral ischemia and promotes improvement in spatial learning and memory dysfunction. So tacrolimus may be a candidate for treatment of ischemia brain damage.