1. Background
The role of sensory-motor system in semantic representation is highly controversial. So far various theories try to address the issue (1). Theories about strong embodiment claim that low-level sensory and motor information is activated in primary cortical areas as part of semantic processing (2-4). For example, Gallese and Lakoff proposed that the same neural substrates are used for perceiving/doing, imaging, and language comprehension (5). This means that the primary motor cortex (M1) plays similar roles in action language comprehension, motor imagery, and action execution. Different from strong embodiment, weak embodiment claims that the sensory-motor system can partly contribute to semantic processing (6, 7). Moreover, semantic representation is different from raw sensory-motor experiences, and hence the roles of in action language processing are different in motor imagery and action execution.
Several studies have indicated that M1 is activated during action language comprehension (8-13), and these findings support the strong embodiment view that raw sensory-motor information is used to represent semantic information. However, researchers also have found that the involvements of sensory-motor areas in action language comprehension and motor imagery are different. For instance, Willems et al. showed that in a motor imagery task, manual verbs elicited stronger activities in the primary motor cortex than did non-action verbs, whereas in a lexical decision task the manual verbs merely showed stronger effects in areas of premotor cortex. In addition, there was no overlap or correlation between the premotor effects elicited by the two tasks (14). The results suggest that the motor system plays different roles in action verb processing and motor imagery: in motor imagery the motor system is responsible for motor planning and execution, but in action verb comprehension the motor system might be only responsible for motor planning.
The above studies focus on the extent of brain activations in the motor system during action language comprehension and motor imagery, but whether interaction within the motor system is modulated by task demand (e.g., action verb processing, motor imagery, and hand motion execution) is still unclear. One promising approach to address the issue is effective connectivity analysis, which computes a directional influence of one neural system over another (15).
So far there are several methods to achieve effective connectivity analysis. One method is hypothesis-driven approaches, such as structural equation modeling (SEM). SEM is a multivariate regression analysis used to detect contemporaneous interactions among variables (16). In SEM, seed regions and their connections are specified by the user, and the path coefficient at each connection and model fit indices are obtained (17). Another method is data-driven analysis, such as Granger causality (GC) (18, 19). In GC, if the past information of a variable X can help predict the future of a variable Y with better accuracy, then the variable X is thought to “Granger cause” the variable Y (19). In fMRI, vector autoregressive (VAR) analysis is used to achieve GC modeling, and the delayed effects of one or multiple lags are used to calculate the temporal and cross-region interactions in a network (17). Compared with hypothesis-driven analysis, the specification of model content in GC lies in the regions of interest (ROIs) involved in a network and the number of lags to use. Once the ROIs are defined, it is the data itself that leads to the statistical inference of temporal and cross-region interactions.
2. Objectives
In the current study, we examined the directional interactions among the supplementary motor area (SMA) and M1 area when participants performed action verb reading, motor imagery and hand motions. SMA is a part of the primate cerebral cortex contributing to the control of movement, and M1 is the main area contributing to movement execution. The two regions have been found strongly involved in hand motion execution. In this study, participants passively read verbs about manual action, performed motor imagery of manual action, and executed manual actions. Anatomical ROIs were selected in the bilateral SMA and M1, and then GC analysis was used to compute the causal influences among the four regions. According to strong embodiment (5), the same neural substrates are involved in perceiving/doing, imaging and language comprehension. Thus, the motor system plays similar roles in action language comprehension, motor imagery and action execution. If this is true, then similar connectivity patterns should be observed in all task modes. According to weak embodiment, sensory-motor information partly contributes to semantic processing, and the motor system might play different roles in action verb processing, motor imagery and action execution. If this is true, then the connectivity pattern in action verb processing might show both similarities and differences with those in motor imagery and hand motion execution.
3. Materials and Methods
3.1. Participants
Participants included 19 Chinese native speakers (7 males, average 24.7 years) who got compensation for the participation. All were right-handed according to a Chinese version of the Edinburgh Handedness Inventory (20). All had normal or corrected to normal vision and none had a history of neurological or psychiatric disease. Each participant provided the written informed consent to the procedures approved by the Imaging Center for Brain Research at Beijing Normal University. The study protocol conforms to the ethical guidelines of the 1975 Declaration of Helsinki as reflected in a priori approval by the institution's human research committee.
3.2. Materials
The detailed information about the stimuli in the passive reading task can be found in (21). Here we provided information relevant to the stimuli used in this study. Both the passive reading session and the motor imagery session contained 48 single-character Chinese verbs about manual actions, such as jiao (stir), sao (sweep), wa (dig). All verbs were rated on familiarity, concreteness and imageability with Likert-like 7-point scales (1 = very low, 7 = very high) and hand action ratio with a dichotomous scale (1 = yes, 0 = no) by 20 native Chinese speakers (none of them participated in the fMRI experiment). The word frequency of each verb was obtained from the Language Corpus System of Modern Chinese Studies (22). Paired t-tests showed that the manual verbs in the passive reading task and the motor imagery task matched well with each other (ts < 1, ps > 0.1) (Table 1).
| Task | Frequency | Familiarity | Concreteness | Imageability | Hand Ratio |
|---|---|---|---|---|---|
| Passive reading | 31.8 ± 30.2 | 5.4 ± 0.8 | 5.2 ± 0.7 | 5.8 ± 0.5 | 0.8 ± 0.1 |
| Motor imagery | 32.6 ± 30.0 | 5.5 ± 0.7 | 5.2 ± 0.6 | 5.9 ± 0.4 | 0.8 ± 0.1 |
a Hand ratio refers to the hand action ratio. Data are presented as mean ± SD.
3.3. Task and Procedure
The fMRI experiment included three separate task sessions: a passive reading session, a motor imagery session, and a hand motion session. To avoid the influences of motor imagery and hand motion on passive reading, the order of the three tasks was fixed: the passive reading session always preceded the motor imagery session (14), and the hand motion session was always the last one.
The procedures for the passive reading session and the motor imagery session were same. The 48 verbs in each session were divided into 6 blocks, and in each block a verb was displayed on the screen for 2 sec plus a .5 sec blank. Each block was followed by a 16 sec rest. Participants were told that in the passive reading task they should read each verb carefully and that in the motor imagery task they should imagine the situation that they were doing the actions described by each presented verb. In both tasks, participants were asked to keep their head, hand and body still.
The hand motion session contained 6 task blocks, and each block lasted 20 sec followed by a 16 sec rest. Within each block, a signal consisted of three asterisks (***) appeared on the screen 10 times to indicate the frequency of hand motion. Participants were instructed to pantomime grasping actions with their left or right hand according to the frequency of the asterisk signal. An instruction appeared on the screen to tell participants which hand they should use at the beginning of each block. The order of hand motion was randomized.
After scanning, participants performed two post-tests. They were asked to select the words presented in the passive reading session from a word list and select pictures that were similar to their imagery from a picture list. All participants could recognize the presented words and pictures describing depicted actions.
3.4. Data Acquisition
Image acquisition was performed at the Imaging Center for Brain Research in Beijing Normal University using a 3.0-T, whole-body MRI system (Siemens, Trio Tim) with a standard radiofrequency head coil. Functional images were acquired with a gradient echo planar imaging (EPI) sequence (FA =90o, TR = 2000 ms, TE = 30 ms, 32 axial slices, thickness = 4 mm, inter-slice gap = 8 mm, voxel dimension = 3.125 × 3.125 × 4.8 mm3, 32 axial slices). After that, high-resolution anatomical MRI for each participant was acquired using a MPRAGE sequence (FA = 7o; TR = 2530 ms, TE = 3.39 ms, 128 sagittal slices, voxel dimension =1.33 × 1.33 × 1.33 mm3).
3.5. Data Analysis
The fMRI data were analyzed with AFNI software package (23). Head motion correction for EPI images was performed with a six-parameter rigid-body transformation after slice timing. Each participant’s anatomical image was coregistered to standard Talaiarach and Tournoux space (24), and the EPI images were aligned to the anatomical image. A 6-mm Gaussian kernel was used to spatially smooth the functional data and all images were resampled to 2 × 2 × 2 mm3 resolution.
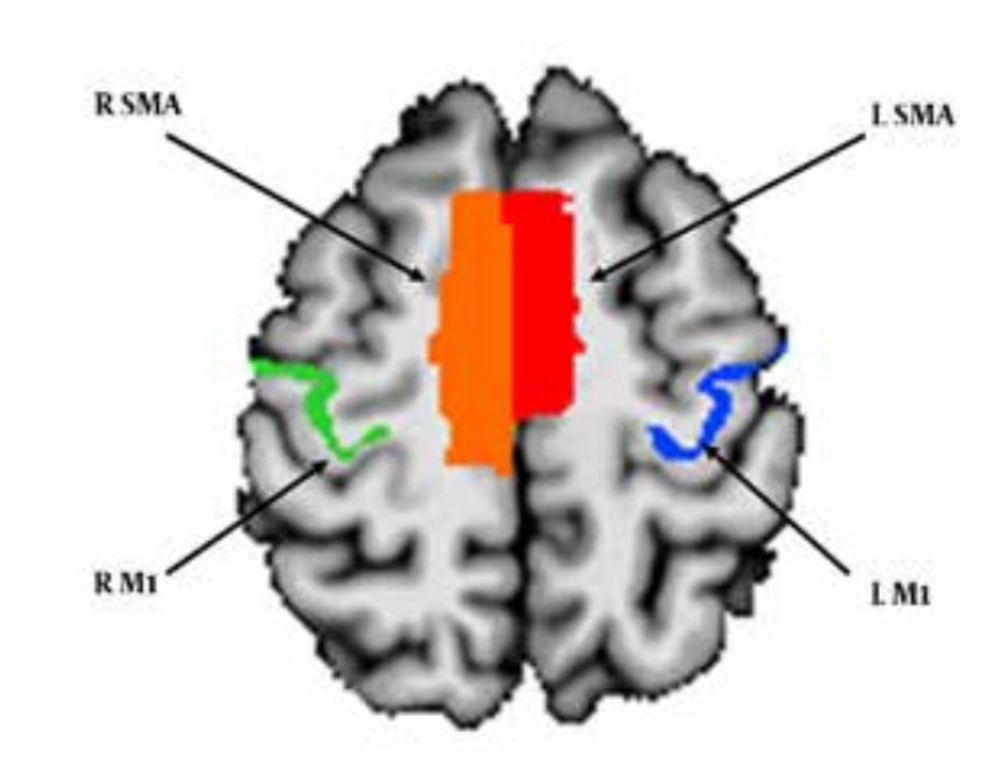
A Granger Causality analysis was conducted to investigate the causal relations among the bilateral M1 and SMA . Anatomical ROIs in the bilateral M1 were selected based on the TT-Damon Template, and anatomical ROIs in the bilateral SMA were selected based on the TT-N27 template (Figure 1). After that, the average time series in each ROI was computed for each participant. 1dGC.R program (http://afni.nimh.nih.gov/sscc/gangc/1dGC) in the AFNI package was employed to conduct the GC analysis. In individual analysis, the averaged time series in each ROI was entered into a VAR model as the input. The model was chosen according to the Akaike Information Criterion. Six head-motion parameters were entered into the VAR model as covariates to minimize confounding effects. For each participant, both the path coefficients and the corresponding t-values for the causal effects among the four ROIs were computed. Group analysis was conducted based on the path coefficients and the t-values from individual analysis. Group t-tests were used to test the significance of the causal relations among the ROIs. The final results were reported at the threshold of P < 0.05 with Bonferroni correction for multiple comparisons.
4. Results
Results are as below:
4.1 Behavioral Results
Accuracies of word recognition and picture recognition were expressed as scores. In the passive reading task, the average d-prime was 2.57 (SD = 0.90). In the motor imagery task, the overall average d-prime was 2.37 (SD = 0.80). No significant task effect was revealed by paired-sampled t-test [t (18) = 0.757, P = 0.458].
4.2. GC Results
4.2.1. GC Results in the Passive Reading Task
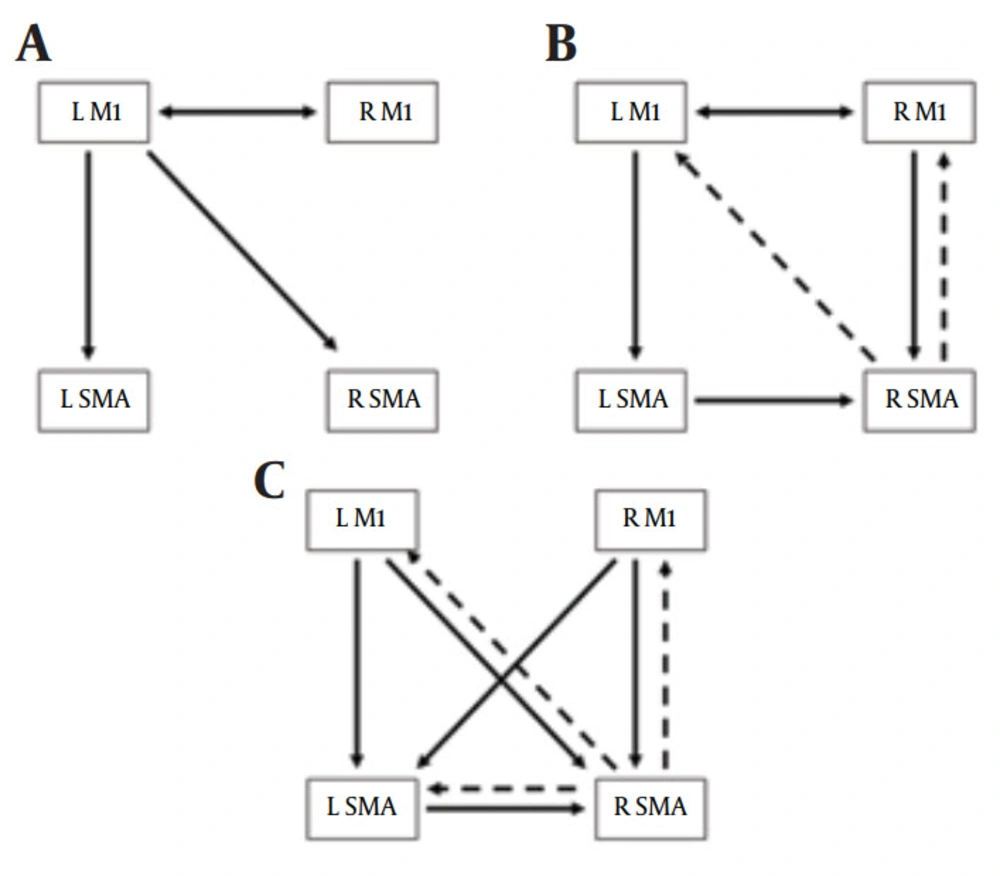
In the passive reading task, the left M1 showed influences on the bilateral SMA, and bidirectional influences was found between the left M1 and right M1 (Figure 2A).
4.2.2. GC Results in the Motor Imagery Task
In the motor imagery task, the causal interactions among the four ROIs were more complicated than that in the passive reading task. The left M1 showed an influence on the left SMA, and bidirectional influences were indicated between the bilateral M1. In addition, the left SMA and the right M1 indicated influences on the right SMA, and the right SMA showed negative influences on the bilateral M1 (Figure 2B).
4.2.3. GC Results in the Hand Motion Task
In the hand motion task, complicated causal interactions were found among the bilateral M1 and SMA (Figure 2C). The bilateral M1 and left SMA showed positive influences on the right SMA, while the right SMA indicated negative influences on the other three regions. Additionally, the bilateral M1 showed influence on the left SMA.
(A) Granger causality results for the passive reading task. (B) Granger causality results for the motor imagery task. (C) Granger causality results for the hand motion execution task. Single directional arrows indicate single directional causal influences, and bidirectional arrows indicate bidirectional causal influences. Solid lines indicate positive influences. Dashed lines indicate negative influences. L = left, R = right, M1 = primary motor cortex, SMA = supplementary motor area.
5. Discussion
The embodied feature of semantic representation is still in controversy. Previous studies have shown different activations in the motor system during action verb processing and motor imagery; however, whether the motor system interaction is modulated by task demand is still unknown. The current study used effective connectivity analysis to investigate the causal interactions among the bilateral SMA and M1 during verb passive reading, motor imagery, and hand motion execution. The results indicated that the complexity of the connectivity pattern changed across tasks: The hand motion task elicited the most complicated network, the motor imagery task elicited a less complicated network, and the passive reading task elicited the fewest connections. A similar connectivity was found between the left M1 and left SMA in all tasks. Additionally, passive reading and motor imagery indicated similar connectivity patterns between the bilateral M1, and similar connectivity patterns between the left M1 and right SMA was revealed in passive reading and hand motion task. Finally, similar negative influences from the right SMA on other regions were revealed in motor imagery and the hand motion task.
The most interesting result is the common connectivity between the left M1 and the left SMA across all three tasks. An influence from the left M1 to left SMA was observed. Previous studies found that SMA is important in storing information necessary for the orderly performance of multiple movements and planning movements ahead (25), and that SMA is strongly activated when participants imagined that they were performing a complex sequence of finger movements (26). The findings suggest that SMA might be involved in ‘high-order’ aspects of motor behavior (27-30), such as the internal generation of complex movements (26, 27, 31). Recent studies also show that SMA activation can be tightly coupled to M1 during externally cued movements (32). In the current study, a directional influence was found from M1 to SMA during three tasks, and this might suggest that the possible sequence of hand movements cued by manual action verbs during passive reading and motor imagery and the sequence information of hand movements in the motion execution task are processed and stored by SMA to generating the plan of incoming movements (25).
Another interesting result about the connectivity between SMA and M1 is that in the motor imagery and the hand motion tasks, causal influences were found from M1 to SMA bilaterally, but in the passive reading task such influence was only found in the left hemisphere. Given the fact that all the participants in the current study are right-handed, the results suggest that motor component in the semantic representation of manual verbs can be body-specific and shaped by actions one has performed (1). This finding is consistent with (14) which found that handedness can influence motor activity during hand verb comprehension. While right-handed participants activated the left premotor areas during lexical decision of hand action verbs, left-handed participants activated the right premotor areas.
Besides the similar connectivity found between left M1 and left SMA in all three tasks, the passive reading task also indicated similar bilateral connectivity patterns between the left M1 and right M1. But such connectivity was not revealed in the hand motion task. This difference might relate to the stimuli used in the experiment. In both the passive reading and the motor imagery tasks, the manual verbs described complex hand actions. Some of the actions require tool-use, such as qie (cut), kan (chop) and jiao (stir). These complex manual actions can involve both hands, and this might induce the connectivity between the bilateral M1. In the hand motion task, however, the grasping actions performed by participants merely require one hand. Thus, the connectivity between M1 and SMA was observed in each hemisphere, but no connectivity was found between the bilateral M1.
One important difference between the passive reading results and the motor imagery / hand motion results is that the negative influences from the right SMA were only found in the latter two tasks. Several studies have investigated the connectivity between SMA and other motor areas during motor imagery (3, 33), and they have demonstrated that SMA has a suppressive influence on M1 during motor imagery (3, 33). In Solodkin et al., participants performed kinetic imagery (i.e. mental simulation of movement associated with a kinesthetic feeling), visual imagery (i.e. visual representation of their moving limbs) of manual actions and executed manual actions. The structural equation modelling results showed that the connection from SMA to M1 became suppressive during kinesthetic motor imagery (33). This result suggests a physiological mechanism through which the motor system prevents overt movements. Kasess et al. utilized dynamic causal modeling to determine the effective connectivity between SMA and M1 and they found a strong suppressive influence from SMA to M1 in the motor imagery condition (3). This finding indicated that SMA is important for the preparation and suppression of movements. In the current study, both motor imagery and motion execution tasks showed that the right SMA had suppressive influences on the bilateral M1. This is consistent with previous findings that SMA in each hemisphere is reciprocally connected and projects to both contralateral and ipsilateral M1 (34, 35), and that SMA can operate bilaterally (36). However, the suppressive influences from SMA were only found in the right hemisphere, suggesting that the bilateral SMA might play different roles in motor execution and motor imagery. Another result that might support this view is that in the motor imagery and motor execution tasks, the right SMA received positive influences from other seed regions including the left SMA (Figure 2B and 2C). Previous studies have found that when participants had a hand preference, the involvements of the bilateral SMA during hand or finger movements can be different (37). Given the fact that all participants in this study were right-handed, whether handedness influences the role of the bilateral SMA in motor execution and motor imagery needs further investigation.
One methodological issue in the current study is the GC modeling used for computing effective connectivity. Smith et al. claimed that hemodynamic variability between different brain regions may swamp any causal lag in the underlying neural time series, and thus cause bias in lag-based causality analysis (e.g., GC analysis) (38). According to Roebroeck et al., one possible approach to exclude the confound effect caused by systematic difference in the hemodynamic lag at two regions is to show the influence varies in different experimental conditions or cognitive contexts (39). In the present study, we found that the causal interactions varied in different cognitive contexts (i.e. verb passive reading, motor imagery and motion execution) and thus the current result cannot be interpreted merely by the hemodynamic variability between SMA and M1. However, there is still an issue that in GC analysis the instantaneous correlation among regions in a network is regarded to be irrelevant (17), and thus the information of the contemporaneous interactions among the bilateral SMA and bilateral M1 were lost. Future work should combine GC analysis with connectivity analysis focusing on the instantaneous correlations between regions to explore the causal influences within the networks for different task demands.
To summarize, the current finding indicate that although the motor network involved in action verb processing shares similar interactions with those in motor imagery and hand motion execution, the network is less complicated. This result pattern suggests that semantic representation might share some common features with motor imagery and motor execution, but the neural mechanisms under these processes are different. The current result supports weak embodiment which claims that the motor system can be partly involved in and contribute to the semantic representation of action language processing, but semantic representation is different from the raw sensory-motor experiences supported by the primary cortical areas (6, 7).