1. Background
To achieve a suitable pharmacotherapy strategy in epileptic patients, evidence-based study regarding the drug prescription seems to be important. The consequences of case classification and judgment are not only difficult for clinical supervision, but also need to consider plenty of vigilance on patients' care.
The clinical case definition of antiepileptic drugs (AEDs), must comprise such factors as interference of each drug, their significant results, monitoring signs of achievement or breakdown, number of the drugs, which should be tried, accounting seizure incidence, and the role of adverse effects and acceptability.
Alternatively, the philosophy of evidence-based pharmacotherapy (EBP) is to provide a methodical advance in integrating the best obtainable evidence into the procedure of quantifiable judgments for individual patients (1-6). The prescription of AEDs is supported by the recognition of their efficiency and side effects. Because of the comparable efficacies between AEDs, patient's management is distinguished by side effects. Dissimilarities in categorization of side effects, and inconsistency within epileptic population make it difficult to use information on side effects and decide on suitable AEDs.
Decreasing glutamate excitation (Glu) and increasing γ-aminobutyric acid (GABA) inhibition are parts of the mechanisms of action for AEDs. Ionic channels could be potentially accountable for their action too. Carbamazepine, phenytoin, primidone, oxcarbazepine, and possibly sodium valproate affect sodium channels and potentiate GABA receptors. Valproic acid and gabapentin increase the formation of GABA. Gabapentin action may be associated with an active carrier system in the neurons (7-16).
Lamotrigine affects sodium-channels by extending inactivation and though it is less capable in maintaining acetylcholine or GABA, it prevents the release of glutamate and aspartate (7, 17). Topiramate releases antagonism of the excitatory receptor (Glu), blocks the action potentials in a time-dependent behavior, hence, increases the action of the GABA and chloride channels (7, 18).
Clonazepam supports GABAergic in the brain (7, 19). Levetiracetam blocks nerve transmission across synapses by linking to vesicle synaptic protein (7, 20). The mechanism of action for clobazam is associated with GABA receptor (7, 21). Phenobarbital potentiates the effect of GABA at receptor site and also blocks a subtype of glutamate receptor 0020 (7, 22). Zonisamide enhances GABA discharge, inhibits Glu release, voltage-gated sodium channels, and T-type calcium channels (7, 23).
Common adverse reactions of AEDs are vertigo, sleepiness, cerebral deliberating and hematologic adverse effects such as purpura, anemia, thrombocytopenia, lymphadenopathy, increased white blood cell count, lymphocytosis, non-Hodgkin's lymphoma, and increased bleeding time. Other side effects may include agranulocytosis, aplastic anemia, leucopenia, and thrombocytopenia (9, 24-26).
2. Objectives
Administration of AEDs among Iranian epileptic population maintains to increase. In order to achieve drug management, systematic studies on evidence-based pharmacotherapy is important.
3. Patients and Methods
A cross-sectional study was carried out on 24 patients (11 females and 13 males) with a mean age of 27 years (ranged, 7-74 years), registered at the Isfahan Kashani Epilepsy Ward affiliated to Isfahan Neurosciences Research Centre (INRCC). There was no induction in treatment procedure. The study was approved by the Institute Research Ethics Committee (IREC). According to AEDs monotherapy or polypharmacy, two groups were made. Group one included patients under one AED (monotherapy) therapy, and the second group consisted of patients treated with more than one AED (polypharmacy). Sex, age, time of seizure onset, biochemical and hematological parameters, types of AEDs, and number of drugs used by each patient were recorded in a d-base. Descriptive statistics such as mean, minimum, and maximum was calculated for variables of interest using SPSS application for windows.
4. Results
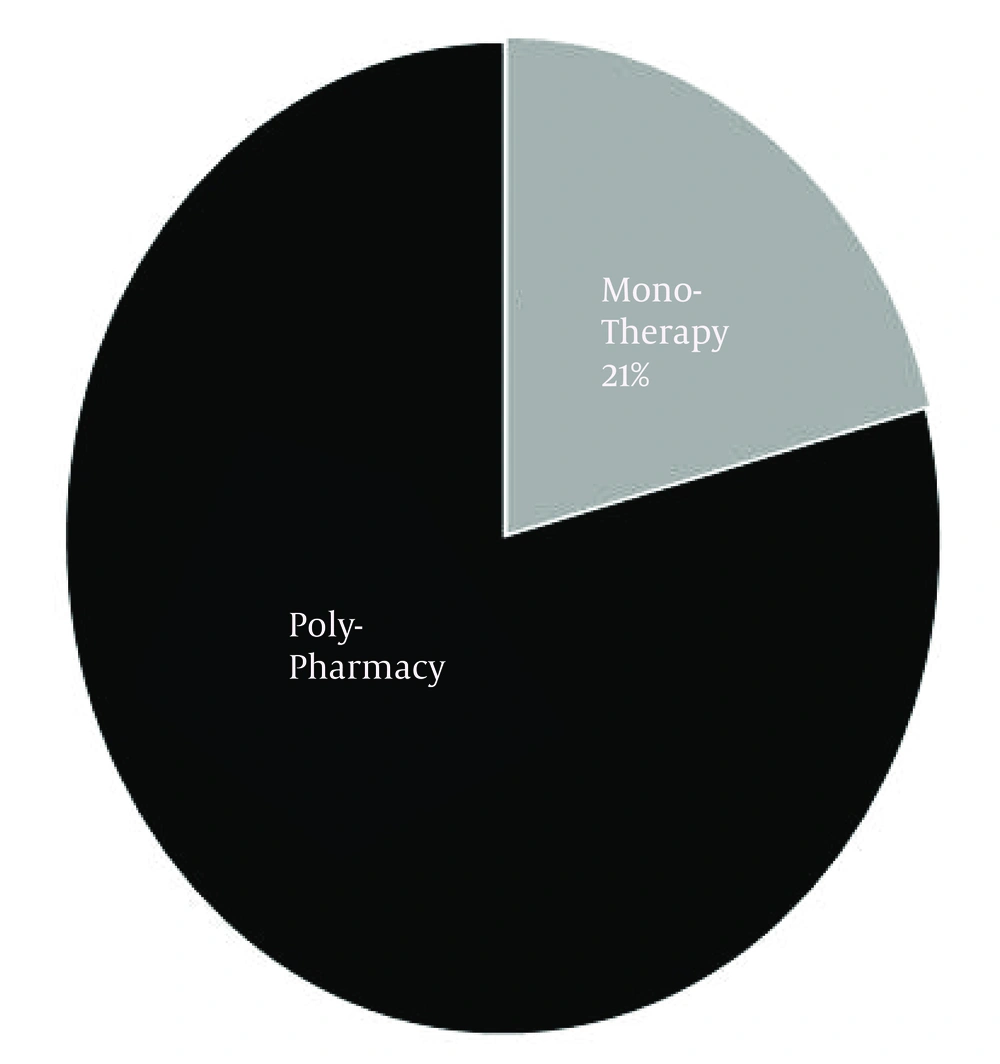
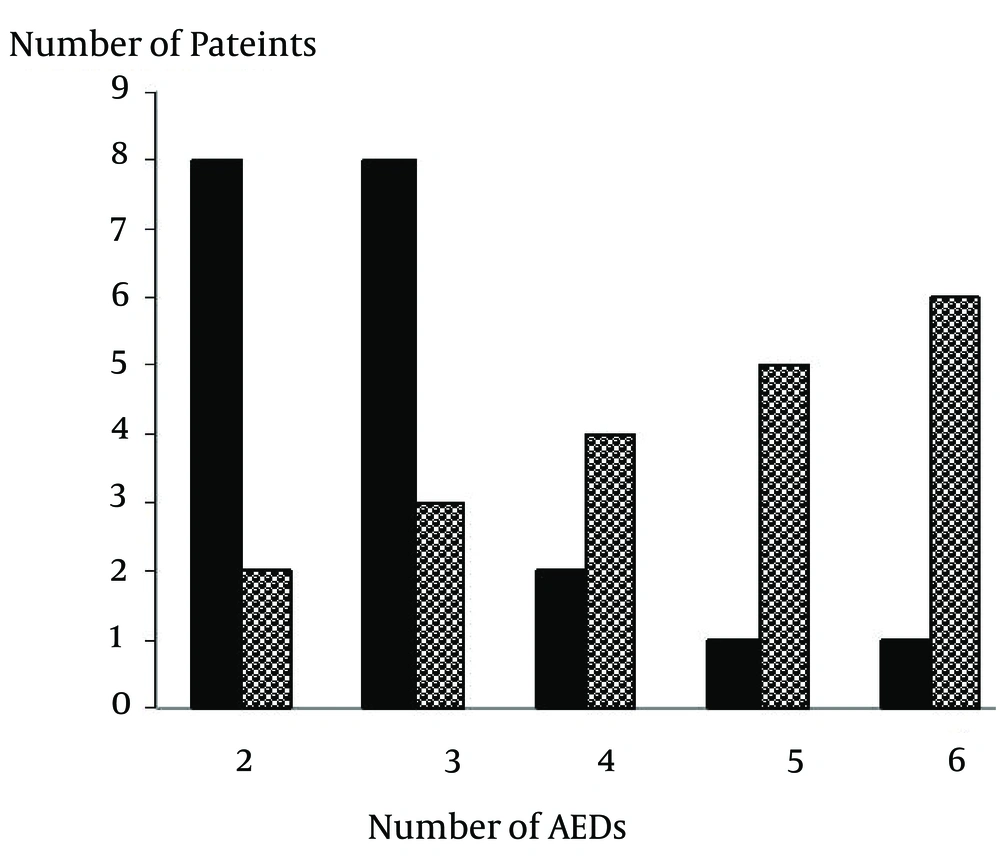
The number of AEDs used by each patient ranged from 1 to 6 with a mean of 3. Within the studied population, the incidence of polypharmacy was 79% (Figure 1). Figure 2 shows that the prescription of AEDs in 50% of patients consisted of 3 to 4 drugs.
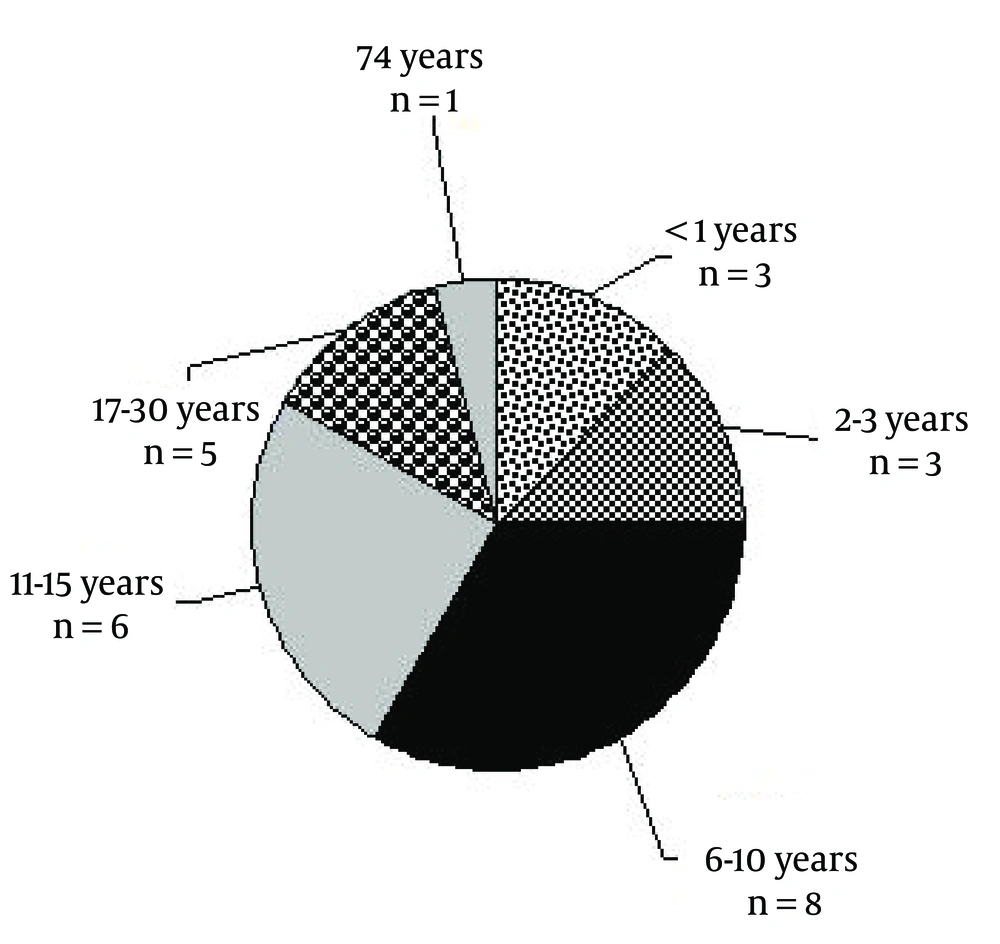
The mean onset of the first epileptic attack was at 11.6 years old, ranged from the beginning of the life to 74 years old. Figure 3 shows the distribution of the onset of first seizure attack.
In 70% of the studied population, the time of seizure onset was under 16 years old. Around 14 varieties of AEDs included both generic and trade names that were used. Valproic acid (Depakote), carbamazepine (Tegretol), phenytoin, lamotrigine, topiramate, clonazepam, levetiracetam, clobasam, primidone, zonisamide, phenobarbital, ethosuximide, gabapentin, oxcarbazepine were the best-selling drugs. Table 1 shows evidence-based pharmacotherapy in a number of studied epileptic patients.
| 562; VP 563, 567, 569; CBZ 576; PHT, Treatment Follows Gold Standard Monotherapy | ||
|---|---|---|
| Patient’s code No. | Type of AEDs administered for Each patient, varieties of AEDs in Each Prescription | Predicting AEDs Mechanism of Action in Combination Therapy (1-48) |
| 554 | CBZ, PID + VP, PIH + Zoni | CBZ; Decrease firing in sodium channel, potentate, GABA receptor, VP; possibly affect sodium channel, increases the formation of GABA, Zoni; inhibits Glu release, inhibits voltage-gated Na channel, inhibits T-type calcium channel |
| 556 | CBZ + VP + LAMO | LAMO; Extended inactivation affects sodium channels, is less capable in preserving acetylcholine |
| 557 | TOP + VP + PHENO | TOP; blockade the action potential, GABA action↑, controls chloride channel, release antagonism of the glutamate excitatory amino acid receptor, PHENO; GABA receptor affinity, a subtype of glutamate receptor blockage |
| 559 | PHT/LEV | PHT; sodium channel by decreasing firing, potentiate GABA receptor, LEV: blocks nerve transmission |
| 564 | CBZ/PHT/GBP/CLON/LAMO | CLON: support GABA-ergic situated in the brain |
aAbbreviations: CBZ, carbamazepine; CLON, clonazepam; LAMO, lamotrigine; LEV, levetiracetam; PHENO, phenobarbital; PHT, phenytoin; PID, potent inductor; PIH, potent inhibitor; TOP, topiramate; VP, valproic acid; ZONI, zonisamide.
Patients with code numbers 562 (on Depakote), 563, 567, 569 (on carbamazepine), and 576 (on phenytoin) received gold standard monotherapy. Prescriptions were based on two AEDs in patients with code numbers 559 (phenytoin, levetiracetam), 565 (carbamazepine-phenytoin compound), 566 (Depakote, lamotrigine), 570 (carbamazepine, valproic acid), 580 (primidone, valproic acid), 582 (carbamazepine, lamotrigine), and 584 (Tegretol, Depakote). Prescriptions were based on three AEDs in patients with code numbers 554 (carbamazepine and Tegretol, valproic acid, zonisamide), 556 (Tegretol, Depakote, lamotrigine), 557 (topiramate, valproic acid, phenobarbital), 568 (carbamazepine, valproic acid, zonisamide), 571 (carbamazepine, valproic acid, lamotrigine), 583 (primidone, phenytoin, topiramate), 585 (Tegretol, topiramate, Depakote) and 586 (valproic acid, ethosuximide, phenobarbital). In two patients, prescriptions were based on 4 and 6 AEDs: patient with code No. 575 (carbamazepine, topiramate, clobasam, lamotrigine) and in another one with code No. 587 (oxcarbamazepine, phenytoin, gabapentin, valproic acid, clonazepam, lamotrigine).
Table 2 shows the mean, minimum and maximum values of biochemistry and hematology parameters within two groups of epileptic patients. With a high mean value of white blood cell count, the maximum values in both groups (9300 monotherapy; 10200 polypharmacy) were high. Red blood cell count, hemoglobin and hematocrit in patients with more than one AED were significantly lower than patients under monotherapy. With a mean value of 43.2 and a maximum value of 84, lymphocyte was significantly higher in group two. With a mean value of 252.3, the maximum value in group two was 559. Blood sugar, with a range of 60-112 mg/dL, does not seem to be significantly different in both groups.
| Serum Biochemical Parameters, Normal Ranges | Group 1 vs. Group 2 (Mean, Min, Max) | ||
|---|---|---|---|
| White blood cell, 4400-11000.mm3 | 6420 vs. 5568 | 3800 vs. 3200 | 9300 vs. 10200 |
| Red blood cell, M: 4.5-5.9 m/mm3, F: 4.1-5.1 m/mm3 | 5.24 vs. 4.50 | 4.13 vs. 3.64 | 6.72 vs. 5.76 |
| Hemoglobin, M: 14-17.5 g/dL, F: 12.3-15.3 g/dL | 13.22 vs. 12.45 | 11.4 vs. 10.4 | 15.1 vs. 14.3 |
| Hematocrit, M: 41.5-50.5%, F: 35.9-44.9% | 40.88 vs. 37.9 | 35.1 vs. 33.8 | 44.9 vs. 43.2 |
| Platelets, 150000-450000/mm3 | 211333 vs. 212812 | 174000 vs. 112000 | 238000 vs. 330000 |
| Lymphocyte, 20-40, % | 32.9 vs. 43.2 | 24.8 vs. 26.2 | 37.4 vs. 84 |
| Sodium, 135-145, meq/L | 140.2 vs. 139.7 | 136 vs. 135 | 143 vs. 144 |
| Potassium, 3.5-5, meq/L | 4.2 vs. 4.01 | 4 vs. 3.5 | 4.3 vs. 4.6 |
| Blood urea nitrogen, 8-24 mg/dL | 14.2 vs. 12.42 | 11 vs. 9 | 18 vs. 17 |
| Calcium, 8.2-10.6, mg/dL | 8.5 vs. 8.7 | 8.2 vs. 8.0 | 8.9 vs. 9.3 |
| Phosphorous, 2.5-4.5, mg/dL | 4.7 vs. 4.07 | 3.6 vs. 2.5 | 6.7 vs. 5.4 |
| Blood sugar, 70-110, mg/dL | 84.4 vs. 89.1 | 70 vs. 60 | 110 vs. 112 |
| Alkaline phosphates, 60-360 u/L | 163.6 vs. 252.3 | 2.4 vs. 87 | 328 vs. 559 |
| Aspartate aminotransferase, 4-40 u/L | 25.3 vs. 19.2 | 10 vs. 12 | 39 vs. 42 |
5. Discussion
Controlled monotherapy could be used as the usual pharmacotherapy strategy for most of the epileptic cases, but interindividual and intraindividual susceptibility appear to be more significant than the number, form the perspective of AEDs. According to previous researches, alternative monotherapy or early treatment with another AED could result in similar efficacy. In this regard, side effects might be associated with monotherapy or combined therapies (29).
While the incidence of monotherapy within the studied population in this article was around 21%, there is sufficient data that AEDs side effects in both forms (monotherapy or polytherapy) could influence negatively patients' quality or quantity of life. Many previous studies reported that up to 50% of patients with epilepsy were treated at least intermittently with more than one AED. In these cases, identification of a specific genetic cause could have a significant personal value, even in the absence of clear clinical utility (30-32). Assortment of AED to control epileptic attack should be based on several factors, including mechanism of action, side effect profile, and AED-AED interactions (7, 33). For example, co-prescription of valproic acid with lamotrigine considerably increases severe cutaneous reactions (34).
Treatment of epileptic attack based on monotherapy or rational polypharmacy is not always contradictory, as many anticonvulsants have different mechanisms of action and combination therapy may potentiate their effects (7, 30). Prescribing an ideal combination of AEDs for each patient could be a challenging process. A recent publication confirms that the trace element situation has been considerably changed in both conventional and newer AEDs (35).
The ABCB1-C3435T polymorphism is also likely to act as a risk factor for resistance to AEDs (36). The lack of sufficient data related to AEDs, poverty, cultural viewpoints, debates, poor health communications, and scarcity of skilled experts contribute to the management space (37). Adverse drug reactions such as hepatotoxicity, mitochondrial toxicity, hyperammonemic encephalopathy, hypersensitivity syndrome reactions, neurological toxicity, teratogenicity, metabolic and endocrine adverse events have been reported in relation to valproic acid (7, 38, 39). Valproic acid also reduces alkaline phosphatase and hydroxyproline in lumbar spine (40). Skin reaction to at least one AED such as carbamazepine, phenytoin, lamotrigine or oxcarbazepine has been reported in 30 out of 300 patients; one of them developed Stevens-Johnson syndrome (34, 41-43).
AEDs-drug interactions are often associated with the cytochrome P450 (CYP450) enzymes. This group has more than 50 enzymes; six of them metabolize 90 percent of the drugs, among them the two most significant enzymes of CYP3A4 and CYP2D6. CYP450 can be inhibited or induced by drugs, resulting in clinically significant drug-drug interactions that can cause unanticipated adverse reactions or therapeutic failures.
Standard AEDs doses may cause side effects associated with eminent minimum concentration if a person metabolizes poorly or has a CYP450 enzyme inhibitor added to therapy. Adverse effects are more likely to occur in treatments with AEDs, because these drugs have a narrow therapeutic window and depend on enzyme CYP450 for their metabolism too. Potent inducers of CYP450 such as carbamazepine, phenytoin, and phenobarbital can cause clinically significant drug interaction by increasing enzyme synthesis (7, 44-48).
Finally, to prevent or decrease comorbid circumstances associated with epilepsy management, the alternative process should be based on considering the following guidelines to decrease AED-AED interaction: administration of preliminary dose, stabilizing drug level (minimum therapeutic concentration), increasing dosage, monitoring frequent and severe side effects, and predicting pharmacokinetics behavior.