1. Background
Despite many developments in the fields of infectious diseases treatments, central nervous system (CNS) infections, particularly in developing countries, are still threatening for human health (1). These clinical syndromes include bacterial and viral meningitis, encephalitis and localized infections such as cerebral abscesses, subdural empyema, epidural abscess and infectious thrombophlebitis (2, 3). The causative agents of CNS infections may be varied based on age, geographical region, concomitant diseases and the route of exposure (4, 5). Most frequent germs responsible for acute bacterial meningitis in healthy adults contain Streptococcus pneumonia (50%), Neisseria meningitides (25%), group B Streptococci (15%), and Listeria monocytogenes (10%). Haemophilus influenzae is involved in less than 10% of the cases (3). The clinical signs may develop as a progressive acute form during some hours, or appear as a subacute form which may be exacerbated after some days. The classic triad of meningitis includes fever, headache, and neck stiffness, and more than 75% of patients demonstrate some degrees of altered consciousness status from lethargy to coma. Ninety percent of cases have two of these four symptoms (6). A definite diagnosis is ascertained by clinical findings and CSF analysis, in which pleocytosis of 10 to 10000 cells per μL, mostly with neutrophil dominance and elevated protein content and decreased glucose level is observed. There is no comprehensive information about meningitis incidences in Iran except for those limited researches conducted on small populations (7). The meningitis outbreaks are mostly reported in dry seasons and low temperatures, which may be a cause of human accumulation in some areas (8). Aseptic meningitis is a benign syndrome in which pyogenic bacteria have not any role and occurs with infectious and noninfectious causes, more commonly in children (9). The disease occurs in about 75000 individuals annually, more frequently in summer and early fall (3, 10). In the viral encephalitis parenchyma is involved, but it is often concurrent with meningitis (meningoencephalitis). Moreover, it has an incidence of about 20000 people every year, and herpesviridae family is the most important cause of sporadic encephalitis. Altered consciousness, behavioral and mental disorders, focal nervous deficits, and also focal and generalized seizures are more routinely observed in this type of infection rather than the bacterial form (3). Electroencephalography and cerebral imaging techniques, particularly MRI can be useful to confirm the diagnosis (3, 11, 12). The gold standard method of diagnosis is PCR of cerebrospinal fluid (3, 13). Cerebral abscess shows the clinical signs of acute or chronic headache (the most frequent), and focal nervous deficits (depending on where in the brain it is located), while nausea, vomiting, seizures, and consciousness alterations may be obvious as well. Cerebral abscess is rarely observed (0.3 - 1.3/100000) in which the most common cause is Streptococci species (3). MRI and CT scan may be regarded as the main diagnostic procedures, which show a mass and edematous area. The parietal and temporal lobes are mostly affected (14).
2. Objectives
This was a retrospective study on computerized medical records of patients admitted in a referral hospital with impression of CNS infection, and it aimed to indicate the epidemiological, clinical, and paraclinical characteristics of patients with various CNS infections in this geographic region (north of Iran), to approach a prompt diagnosis and treatment. We had some limitations in this study as follows: extracting data from one hospital relying on medical records, and lack of previous study about CNS infections in this center, but this study would help to find the risk factors for development of CNS infections in our referral center based on data collected about risk factors and epidemiologic, clinical and laboratory features of CNS infections to facilitate prompt diagnosis and treatment of future patient..
3. Patients and Methods
The current descriptive retrospective study, under study population included the computerized medical records of patients hospitalized in infectious diseases department, general internal medicine department, and pediatrics section of Qaemshahr Razi hospital between 2008 and 2012 due to different infections of CNS. Inclusion criteria were as follows ; patients who had the related signs, including fever, headache, nausea and vomiting with a rapid onset, and also showed a pleocytosis in CSF study with neutrophil dominance, low glucose and elevated protein, and positive results for bacterial culture or smear,. In the cases with negative results for smear and bacterial culture, the diagnosis was established based on clinical findings and CSF analysis. Aseptic meningitis diagnosis was established based on the above-mentioned clinical findings accompanied with normal glucose level and protein content, and lymphocytic pleocytosis of CSF. Also, diagnosis of viral encephalitis was established based on a combination of clinical findings (including a body temperature greater than 38 degrees for less than seven days, and at least one of the three signs of (a) neck rigidity, Kernig's or Brudzinski's signs of meningitis, (b) altered consciousness, and (c) new occurrence of seizure, CSF analysis (as lymphocytic pleocytosis), normal protein and glucose levels, and cerebral MRI which showed a unilateral or bilateral temporoparietal region (for herpes encephalitis). Furthermore, diagnosis of cerebral abscess was based on clinical findings including fever, and the signs of a space-consuming cerebral lesion combined with lesions distinguished by CT or MRI. An informational form was filled by physicians or nurses at admission time, in which the epidemiological and demographic characteristics, chief complaint, clinical features of the disease, a history of previous diseases, administrated drugs, a review of medical events in the patient's family, duration of hospitalization, laboratory and radiological findings, and some others were included. The patients' information was next extracted from their files and recorded in specific forms. The statistical analysis was performed using SPSS version 13. To achieve this, some frequency and descriptive tests were applied. The t-test was applied to evaluate and compare the quantitative data and means. In addition, chi-square and fisher exact tests were used to assess the categorized qualitative data. P < 0.05 was considered as significant.
4. Results
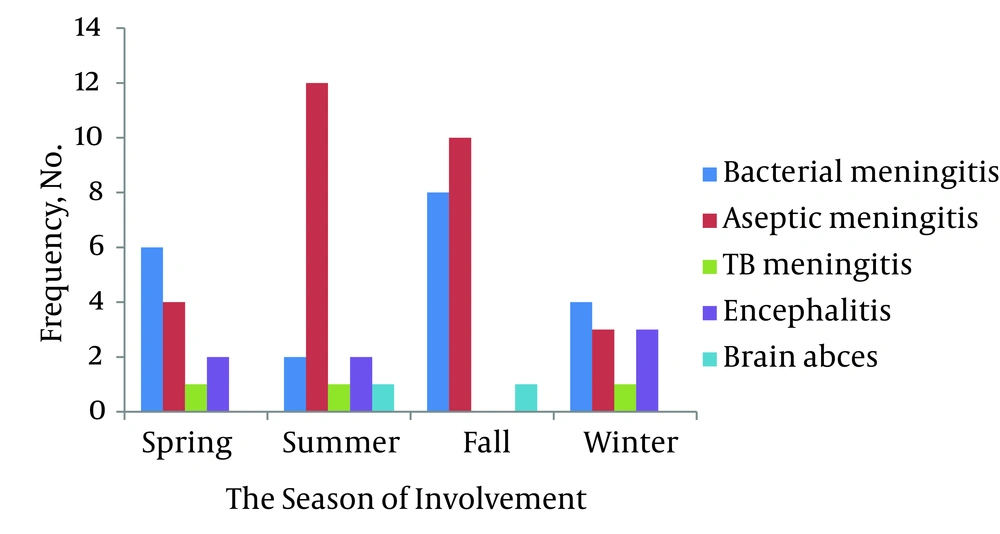
A total of 66 patients affected with CNS infections were evaluated. Of 16532 hospitalized patients with infectious diseases in Razi Hospital, 0.4% cases were involved with CNS infection, including 13 women (19.7%) and 53 men (81.3%), from which, 57 cases (86.4%) had meningitis, seven (10.6%) encephalitis, two (3%) cerebral abscess, and no cases of epidural abscess, subdural empyema, and infectious thrombophlebitis were founded. Aseptic meningitis (43.9%) and then bacterial meningitis (30.3%) were the most frequent infections, respectively, of which, the aseptic meningitis was the most common infection in both sexes (50% in women and 44.2% in men), but sex had no effect on the type of infection with confidence of 95% (P > 0.05). the average age of patients was 20.7 ± 26.3 (ranged between 18 days and 82 years) (Table 1). The respective average age of women and men were 28.1 ± 29.8 and 25.9 ± 18.3, which had no significant difference with each other (P > 0.05). The prevalent age group (35.9%) was 11 to 20, while the age of 80 included the least frequency (16%). In general, 70.3% (45 individuals) were younger or equal to 30 years. All the infections in patients younger than one year belonged to the aseptic meningitis. Also, most infections (64.4%) resulted to hospitalization of patients aged up to 30 belonged to the mentioned disease, while bacterial meningitis was mostly seen in people between 30 and 60 (61.5%), and eventually, six cases over 60 years old were equally infected with bacterial meningitis, herpes encephalitis, and cerebral abscess. The age groups were significantly effective on the type of infection (P < 0.05). 32.8% of the infections occurred in autumn (Figure 1). The most frequent infection occurred in autumn and summer (47.6% and 66.7%, respectively) was aseptic meningitis, while it was bacterial meningitis in spring and winter (46.2% and 33.3%, respectively). One patient, infected with HIV virus, had concurrent cryptococcal meningitis as well, and one case which had undergone radiotherapy of head and neck following surgery due to cerebral cysts, was affected with abscess. Moreover, two cases (1%) died during their treatment, of which, one had tuberculosis meningitis and the other one had bacterial meningitis, and both were men aged 41 - 50. The average duration of hospitalization was 12.3 ± 10.1 for all patients (Table 1). Sixty four percent of patients were residents of cities, but the area of residency had no significant effect on the type of infection (P > 0.05). The most common complaints mentioned by the patients included fever and headache (30.2%), and then decreased consciousness was in the second order (17.5%).
| Cases, No. | The Most Common Season (%) | Age, Mean ± SD | Start of Clinical Sign to Admission Time, Mean ± SD | Hospital Admission Days, Mean ± SD | The Most Common Complaints (%) | |
|---|---|---|---|---|---|---|
| Bacterial meningitis | 20 | Fall (40) | 23.1 ± 17.8 | 2 ± 1.8 | 10.9 ± 4.5 | Fever and headache (35) |
| Aseptic meningitis | 29 | Fall (40) | 13.4 ± 8.6 | 2 ± 1.4 | 8.8 ± 3.8 | Fever and headache (41.4) |
| TB meningitis | 3 | Spring, summer and winter (33.3) | 33.7 ± 16.2 | 14 ± 7 | 41.7 ± 25.3 | Headache (66.7) |
| encephalitis | 7 | Summer (50) | 57.8 ± 23.4 | 2 ± 1.1 | 6.3 ± 0.6 | Decreased level of consciousness (75) |
| Brain abscess | 2 | - | 66.5 ± 4.9 | 25 ± 18 | 10 | Headache (100) |
| Total | 66 | Fall (32.8) | 26.3 ± 20.7 | 3.4 ± 1.2 | 12.3 ± 8.1 | Fever and headache (30.2) |
Demographic and Clinical Characteristics of Hospitalized People Infected With Various CNS Infections
The most common observed infection in the study population was meningitis with the frequency of 83.3% (53 cases), from which aseptic meningitis was in the first order of frequency and then it was bacterial meningitis (Table 1). Sixty six percent of the patients (44 cases) with meningitis were men. Most cases of meningitis were hospitalized (36.4%) in autumn. Three cases of cryptococcal meningitis (5.4%), three cases of tuberculosis meningitis (5.4%), and one case (1.8%) of each influenzae meningitis and brucella meningitis were reported as well. Also, the most common complaints of the referred patients affected with either bacterial meningitis or aseptic meningitis were fever and headache (with a respective percentage of 35% and 41.4%). However, 30% of the patients with bacterial meningitis had been referred due to a decreased consciousness, and 24.1% of aseptic meningitis infected people had been referred with a complaint of fever. Headache was the most complaint of patients with tuberculosis meningitis (Table 1). Two patients with meningitis (3.6%) had a history of being infected with meningitis frequently; both had demonstrated a history of craniotomy and insertion of cerebral shunts. Seven individuals (10.6%) were hospitalized due to encephalitis diagnosis, from which four cases (57.1%) were proved to have herpes encephalitis, and no causes were found in three of them. 85.7% of the mentioned patients and 100 of the patients with herpes encephalitis were men. The patients with encephalitis had a main complaint of decreased consciousness (75%) and fever (25%). Table 2 and Table 3 present a report of clinical findings in people with various CNS infections. Fever was the major clinical finding in bacterial meningitis (95%), aseptic meningitis (89.7%), and encephalitis (57.1%), while all the patients (100%) with tuberculosis meningitis had headache.
| Total, n = 66 | Bacterial Meningitis, n = 22, 33.3% | Aseptic Meningitis, n = 29, 43.9% | TB meningitis, n = 3, 4.5% | Encephalitis, n = 7, 18.3% | |
|---|---|---|---|---|---|
| Infection Symptom, No. (%) | |||||
| Fever | 52 (82.5) | 19 (95) | 26 (89.7) | 1 (33.3) | 4 (57.1) |
| Shivering | 24 (38.1) | 9 (45) | 11 (37.9) | 0 | 2 (28.6) |
| Headache | 46 (73) | 13 (65) | 22 (75.9) | 3 (100) | 4 (57.1) |
| Vertigo | 9 (14.3) | 2 (10) | 4 (13.8) | 0 | 1 (14.3) |
| Neck pain | 2 (3.2) | 0 | 2 (6.9) | 0 | 0 |
| Back pain | 2 (3.2) | 1 (5) | 0 | 0 | 0 |
| Nausea | 30 (47.6) | 11 (55) | 14 (48.3) | 2 (66.7) | 1 (14.3) |
| Vomiting | 37 (58.8) | 12 (60) | 18 (62.1) | 1 (33.3) | 2 (28.6) |
| Diarrhea | 2 (3.2) | 2 (3.2) | 2 (3.2) | 2 (3.2) | 2 (3.2) |
| Photophobia | 4 (6.3) | 1 (5) | 2 (6.9) | 0 | 1 (14.3) |
| Blurred vision | 2 (3.2) | 0 | 1 (3.4) | 0 | 1 (14.3) |
| Convulsion | 12 (19) | 3 (15) | 4 (13.8) | 1 (33.3) | 3 (49.2) |
| Sweating | 5 (7.9) | 3 (15) | 1 (3.4) | 0 | 0 |
| Myalgia | 6 (9.5) | 3 (15) | 1 (3.4) | 0 | 1 (14.3) |
| Decreased appetite | 13 (20.6) | 1 (5) | 7 (24.1) | 3 (100) | 0 |
| Sphincters problems | 6 (9.5) | 3 (15) | 1 (3.4) | 0 | 2 (28.6) |
| Weakness and fatigue | 19 (30.2) | 7 (35) | 6 (20.7) | 2 (66.7) | 1 (14.3) |
| Rhino rhea | 11 (17.5) | 3 (15) | 6 (20.7) | 0 | 1 (14.3) |
| Chough | 8 (12.7) | 2 (10) | 2 (6.9) | 1 (33.3) | 2 (28.6) |
| Dyspnea | 4 (6.3) | 3 (15) | 0 | 0 | 0 |
| Imbalance | 1 (1.6) | 0 | 1 (3.4) | 0 | 0 |
| Memory problems | 1 (1.6) | 0 | 0 | 0 | 1 (14.3) |
| Aggression | 2 (3.2) | 0 | 0 | 0 | 0 |
| Restlessness | 11 (17.5) | 5 (25) | 4 (13.8) | 0 | 2 (28.6) |
| Depression | 0 | 0 | 0 | 0 | 0 |
| Psychosis | 2 (3.2) | 0 | 1 (3.4) | 0 | 1 (14.3) |
| Bizarre behavior | 8 (12.7) | 2 (10) | 1 (3.4) | 1 (33.3) | 4 (57.1) |
The Frequency of Symptoms in Patients with Various CNS Infections Hospitalized in Razi Hospital of Qaemshahr From March 2008 to March 2012
| Total, n = 66 | Bacterial Meningitis, n = 22, 33.3% | Aseptic Meningitis, n = 29, 43.9% | TB Meningitis, n = 3, 4.5% | Encephalitis, n = 7, 18.3% | |
|---|---|---|---|---|---|
| Infection Sign, No.(%) | |||||
| Neck stiffness | 42 (66.7) | 17 (85) | 16 (55.2) | 1 (33.3) | 4 (57.1) |
| Kerning's sign | 3 (4.8) | 2 (10) | 1 (3.4) | 0 | 0 |
| Brudzinski's sign | 5 (7.9) | 17 (20) | 0 | 1 (33.3) | 0 |
| Decreased level of consciousness | 27 (42.9) | 12 (60) | 6 (20.7) | 3 (100) | 5 (71.4) |
| Hypotension | 3 (4.7) | 1 (5) | 2 (6.9) | 0 | 0 |
| Tachypnea | 3 (4.8) | 1 (5) | 0 | 1 (33.3) | 0 |
| Tachycardia | 5 (7.9) | 3 (15) | 0 | 1 (33.3) | 0 |
| Skin rash | 4 (6.3) | 2 (10) | 1 (3.4) | 0 | 1 (14.3) |
| Pupil abnormality | 5 (8) | 2 (10) | 0 | 1 (33.3) | 2 (28.6) |
| Papillary edema | 2 (3.2) | 0 | 1 (3.4) | 0 | 1 (14.3) |
| Neurological abnormality in clinical exam | 10 (15.9) | 4 (20) | 3 (10.3) | 2 (66.7) | 1 (14.3) |
The Frequency of Signs in Patients with Various CNS Infections Hospitalized in Razi Hospital of Qaemshahr From March 2008 to March 2012
CSF puncture was performed in four patients (two cases of cerebral abscess and two cases of encephalitis due to the risk of cerebral herniation). Gram staining of CSF was also implemented which showed two positive cases (10%) (gram positive diplococci). CSF cultures had positive results for three cases (15%), all of which were caused by Streptococcus pneumonia. Moreover, CSF PCR implemented in the patient with influenzae meningitis had positive results. Cerebral CT scan was fulfilled for 49 patients (74.2%), and two cases of cerebral edema (10%) were observed in patients with bacterial meningitis. In addition, temporal lobe lesions (hyper intensity of parenchyma) were reported in two of the patients with encephalitis (28.5%). One case of hydrocephalus in tuberculous meningitis (33.3%) was reported. Eighty percent of patients with bacterial meningitis demonstrated leukocytosis, in which 95% had neutrophil dominance, while 71.4% of patients with encephalitis showed leukocytosis, and all of them had lymphocyte dominance. Hyponatremia was obvious in 30% of patients with bacterial meningitis and 33.3% of tuberculosis meningitis.
5. Discussion
CNS may be infected by different infectious factors containing bacteria, viruses, fungi, protozoa, and helminthes. Most patients refer with a clinical feature of fever, headache, and decreased consciousness. In our investigation, headache with or without fever, was the major cause, for which the patients with various types of CNS infections had presented to the hospital. This is in accordance with many other studies like Arda et al. in Turkey (15), Taylor in Vietnam (16), Alavi in Ahwaz (17), and Celal in Turkey (18); however, in some studies as the one conducted by Dash from Emirate (19), headache was not reported as the most common complaint of patients. Nevertheless, it appears that caring about patients who refer because of fever and headache, with a rapid or prolonged onset, may help them to be diagnosed promptly and prevent further complications or death. In the review of Bahador et al. (20) on 126 patients affected with acute meningitis in Kerman (2003), 9.5% had viral meningitis and the rest were diagnosed as bacterial meningitis. Also, in the study implemented by Dash, from a total of 92 patients, 53% had bacterial meningitis and 37% viral meningitis, but our study revealed that 35% of the patients were infected with bacterial meningitis, and 50.8% showed aseptic meningitis (19). This difference, as well as epidemiological aspects, may be due to the fact that our hospital was not the only center in the province where the patients with various CNS infections could refer to, and there were other centers such as a neuroscience specialist's center in which patient reception was performed. Therefore, our study could not be able to evaluate the prevalence of infections occurring in the province and infection types frequency. Mousavi et al. performed a study on hospitalized patients with meningitis in Tehran and reported a higher incidence of disease in autumn and spring, which was in accordance with our study (21). In the mentioned study, the most incidence of infection occurred in males aged below five, while our study demonstrated the most incidence of infection in males aged 11 to 20 years. Generally, the ratio of disease incidence in men to women was 2.1, and the least frequency of the disease was observed in patients aged 15 to 30 in the study of Mousavi, while our results showed this ratio as four, and the least frequency of infection was observed in patients younger than one and older than 50 years. In addition, the most variety in disease frequency was at ages between 11 and 20 in both sexes. Mousavi et al. reported that women are at a risk of meningitis infection 2.6 times more than men, and a higher risk of involvement in lower ages (21). In contrast, the present study showed that men aged 11 to 30 are at higher risk of being infected with meningitis (63.6%). Also, the results of the current study showed positive CSF cultures in 15% of patients with meningitis, and just 10% of the cases had positive results in smears. Additionally, Bahador et al. (20) reported 10.5% positive cultures, and Arda et al. (15) demonstrated positive cultures and smears in 36.7% and 38.6% of the samples, respectively. These undesirable results in the field of bacteriology may be due to various factors such as antibiotic abuses and also inappropriate culturing methods. Hence, more investigations are required to be directed in these fields of research to improve and accelerate achieving CNS infections diagnosis.
Regarding the similarities between CNS infections clinical findings, and also various differential diagnoses, the primary clinical symptoms, risk factors, paraclinical characteristics and an appropriate treatment may lead to a decrease in mortality and complications.