1. Introduction
Papillary thyroid carcinoma (PTC) is the most common thyroid malignancy, accounting for 70% to 80% of all thyroid malignancies. It typically occurs in the middle age with a peak incidence in the third and fourth decades. It is the predominant thyroid cancer in children and individuals exposed to external radiations. In comparison to other differentiated and undifferentiated thyroid malignancies, PTC is characterized by an indolent clinical course. Metastases are uncommon and usually involve regional lymph nodes. The distant metastases, mainly including lungs and bone lesions, are very uncommon. They are detected in 6% to 20% of patients during follow-up (1-3). Brain metastases, with about 1% incidence, suggest an aggressive disease and specifically have unfavorable prognosis of less than a year survival. Hence, early detection and appropriate treatment of brain metastasis is necessary, because it can lead to long-term survival (4-10). We report a rare case of brain metastasis from PTC in a 73-year-old man who presented with sudden loss of consciousness and normal findings on follow-up screening tests, 17 years after primary diagnosis of PTC.
2. Case Presentation
A 73-year-old man presented with sudden loss of consciousness (Glasgow coma scale [GCS], 7), left hemiparesis and anisocoria. He was a known case of PTC diagnosed 17 years before presentation, which had led to a total thyroidectomy. The patient had two neck dissections because of lymph nodes metastases and four courses of cervical iodine treatments, the last of which was 13 months ago. Whole body scan by I131 demonstrated no evidence for residual active thyroid tissue or metastatic disease after the last iodine treatment. Results of recent serum thyroglobulin, thyroglobulin antibody, and thyroid function tests were in the normal ranges.
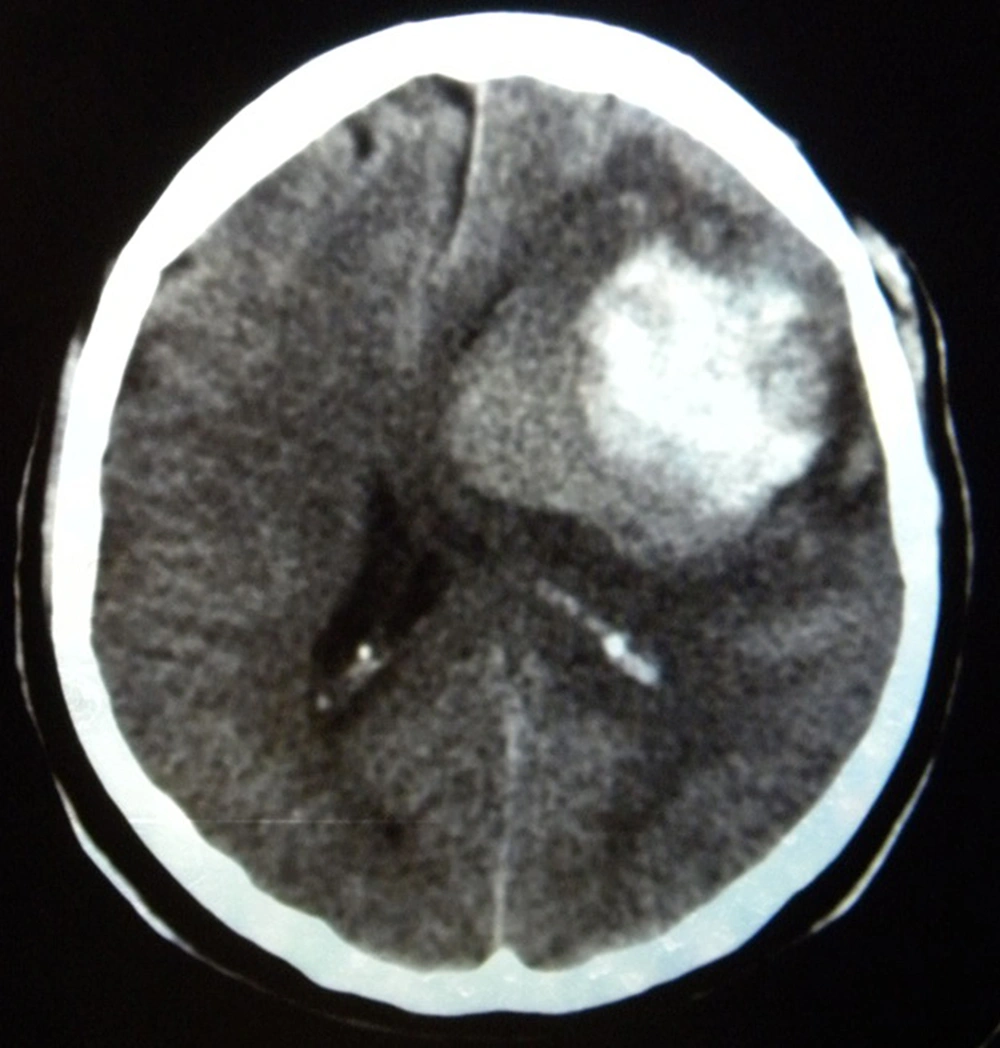
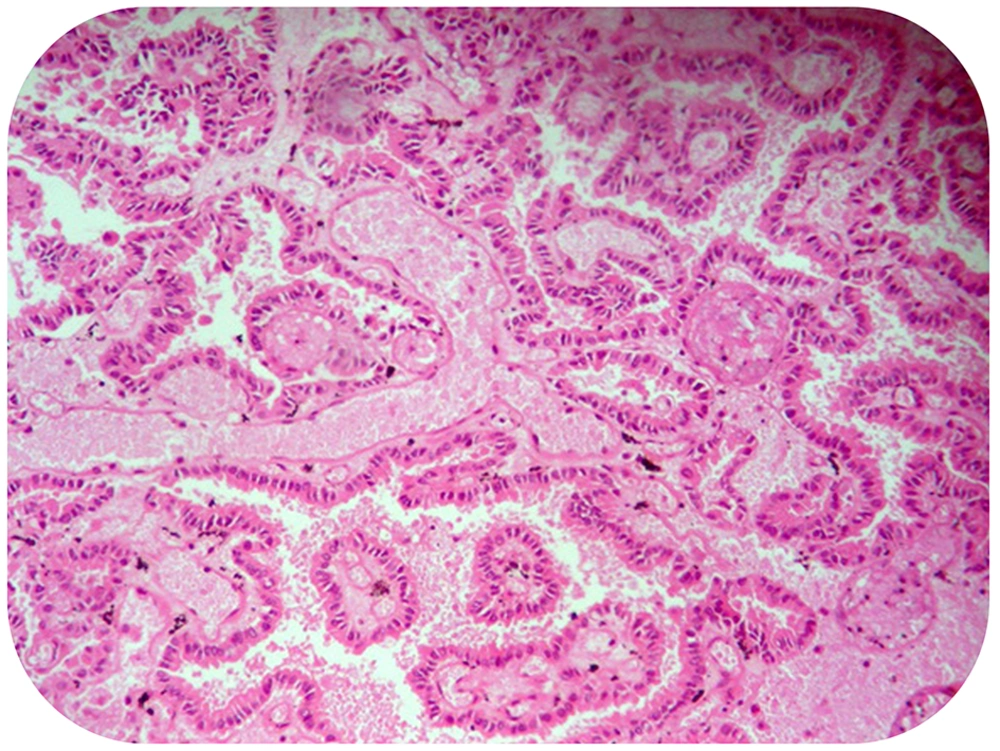
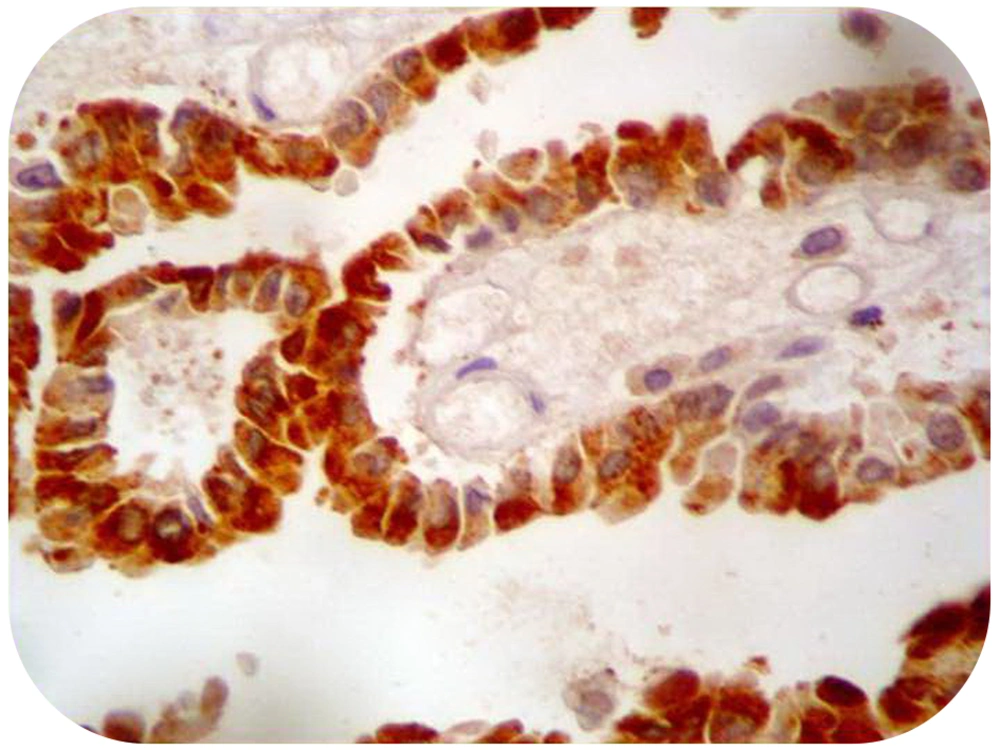
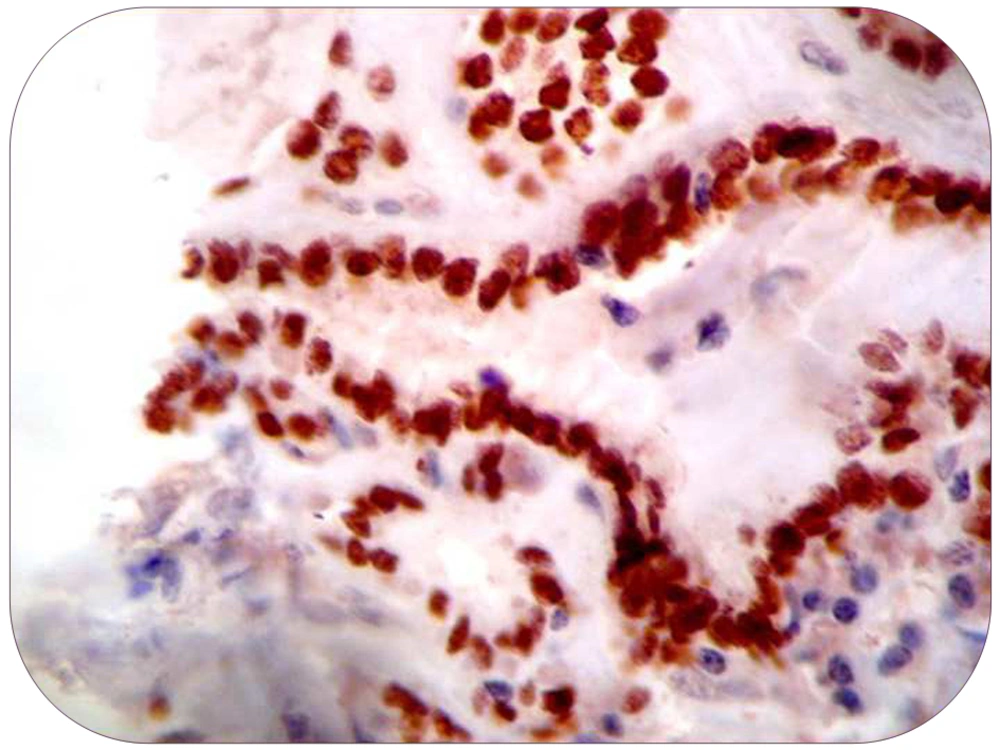
Brain computed tomography (CT) demonstrated a hemorrhagic mass lesion in the left frontal lobe with considerable midline shift (Figure 1). Based on signs of progressive neurologic deterioration referable to the lesion, medically refractory intracranial hypertension, and signs of a mass effect on CT, we decided to operate the patient urgently. A unilateral frontal craniotomy through a curved skin incision was performed and the lesion was addressed via transcortical approach. The hemorrhagic and well-circumscribed lesion was readily separated from cerebral parenchyma and the lesion was totally resected macroscopically. Tissue histopathologic examination revealed metastatic brain tumor of PTC origin, characterized by composition of papillary structures, lined by neoplastic cells with overlapping nuclei (Figure 2). Immunohistochemical analysis confirmed the accuracy of diagnosis (strongly positive reaction to thyroglobulin and thyroid transcription factor 1 [TTF1]) (Figures 3 and 4). Postoperative course was uneventful, and the hemiparesis and loss of consciousness improved.
3. Discussion
PCT is a well-differentiated thyroid carcinoma and a relatively uncommon malignancy that might metastasize. Metastatic lesions are usually seen in the regional lymph nodes and the most common site of distant metastases is the lung, followed by the bone. However, brain metastases are extremely unusual with an incidence rate of 0.15% to 1.3%. In the case of brain metastasis by PTC, cerebral hemispheres are the common site of involvement followed by cerebellum and pituitary gland. Brain metastases are usually asymptomatic, and only a few have suggestive symptoms, including headache, visual disturbances, or ocular motor weakness (1-6). Acute and severe spontaneous cerebral hemorrhage is a life threatening complication that can lead to abrupt onset of neurologic symptoms and rapid death due to intratumoral hemorrhage. Hemorrhagic intracranial metastases represent 3% to 14% of all cerebral metastases and they classically originate from renal cell carcinoma, melanoma, choriocarcinoma, bronchogenic carcinoma, and hepatocellular carcinoma (11-13). The presented case and sporadic cases of hemorrhagic brain metastases caused by PTC, demonstrate that PTC should be considered in the differential diagnosis of hemorrhagic intracranial metastases (11, 13-15). Some risk factors for distant metastasis of PTC include male sex, advanced age, histologic grade, extrathyroidal invasion at initial examination, and multiple organs involvement (16, 17). In general, patients with PTC have an excellent prognosis, with a 10-year survival rate of more than 95%. Several prognostic indicators have been incorporated into various staging systems, including AGES scoring system, MACIS scale and TNM system. Patients with brain metastases are placed in high-risk groups in all staging systems and have an unfavorable prognosis of less than one year (18, 19). PTC has a relatively benign course, and early detection and surgical removal of brain metastasis prolong patients’ survival. Except for the information obtained from the patient’s history, physical examination, and diagnostic studies, there are no definitive methods for diagnosing metastases of this carcinoma to the brain. Only histologic examination provides confirmation of the diagnosis. Screening with I131 is more sensitive than chest X-ray or CT scanning in detecting metastases; however, it is less sensitive than thyroglobulin measurements in detecting metastatic diseases in most differentiated thyroid cancers, except in Hurtle cell tumors (20). Metastatic lesions from differentiated thyroid carcinomas are usually less differentiated than primary tumors. Some metastatic lesions, especially brain metastases, do not have differentiation, leading to no accumulation of I131 in the metastatic sites (21). Salvati et al. reported 12 patients with single brain metastases and none of them had positive findings in I131 whole body scan (22). Fluorine-18-FDG-PET/CT is a sensitive method to detect metastases, which reveal negative results for I131 whole body scan in patients with elevated levels of serum thyroglobulin. However, compared with MRI, Fluorine-18-FDG-PET/CT cannot reveal all brain metastatic lesions; Therefore, magnetic resonance imaging (MRI) is the most sensitive method for detection of brain metastasis (23). Optimal treatment protocol for patients with intracranial metastatic tumors of PTC origin is not clearly defined, and treatment should be tailored to each patient individually. Several treatment modalities with varying results, e.g. surgical resection, gamma knife radiosurgery, whole brain radiation therapy, radioiodine therapy, and chemotherapy, have been used in the limited number of intracranial metastatic PTCs (13, 24-27). Although the presence of a brain metastasis is an overall negative prognostic indicator, surgical resection of brain metastases might help to significantly prolong survival in patients with differentiated thyroid carcinoma, especially in solitary or oligometastatic lesions (13, 24-26). In life-threatening complications such as refractory intracranial hypertension, severe brain edema, tonsillar herniation, and intracranial hemorrhage, surgery is also the treatment of choice (13, 24). However, if a patient is at high risk for surgery or surgery is impossible and in the case of incomplete resection of metastatic lesions or diagnostic biopsy, adjuvant therapy such as whole brain radiotherapy or radiosurgery might be the best therapeutic choice. Whole brain radiation external beam therapy is usually used to treat multiple lesions. There are very few data regarding the efficacy of whole brain radiation therapy after surgery and long-term complications must be considered in patients who are likely to have prolonged survival (24, 27, 28). Gamma knife radiosurgery certainly is an attractive option for the local control of a solitary lesions or a few smaller ones because it is less invasive than craniotomy; it is especially important in patients who might have a limited survival time (24). Patients treated with SRS (stereotactic radio surgery) for brain metastases from primary thyroid cancer have a favorable prognosis with an expected median survival of longer than three years (13, 29). Radioiodine treatment would be a reasonable treatment option if findings of the I131 whole body scan were positive. There have been cases in which a response to such treatment was achieved (24, 27). Chemotherapy has only a limited role in the management of thyroid carcinoma with extracranial metastases (23, 24), and this appears to hold true for intracranial metastases as well (24). Miranda et al. reported a case with ten-year survival (the longest survival of a patient with brain metastases by far), who was managed by combined approach of surgical excision, I131, whole-brain radiation therapy, and gamma knife radiosurgery (30).
Based on a summary of findings from previous case reports, the present case showed some remarkable characteristics. First, the patient presented with acute neurologic deficits due to intracerebral hemorrhage and no other sites of distant tumor dissemination were detected (9, 11, 31). Therefore, surgery could be a more suitable treatment option in case of hemorrhagic metastasis because surgery can relieve neurologic deficits immediately (32). Second, I131 whole body scan demonstrated no evidence of residual active thyroid tissue or metastatic disease, while results of serum thyroglobulin and thyroglobulin antibody were in the normal ranges (1.6 ng/mL and 16.6 IU/mL, respectively). Therefore, we recommend MRI as the most sensitive method for the detection of brain metastases as reported by Xu et al. (23), and it should be taken into consideration in patients with presentation suggestive of brain metastasis, even with normal serum thyroglobulin levels. Third, a combined approach of treatment modalities must be individually modified for each patient.