1. Background
In recent years, increased prevalence and incidence of multiple sclerosis (MS), mainly in females, has been reported (1). Demyelination of axons and loss of oligodendrocyte might be the result of heritable and environmental risk factors (2). The histopathologic change in MS-related trigeminal neuralgia after gamma-knife rhizotomy has been reported recently (3). In females, childhood and adolescence overweight could the exaggerate risk of MS (4). Vitamin D and its receptor (5) are expected to be another risk factor, as in vitro study showed that up-regulation of vitamin D receptor and CYP24A might be induced in primary human astrocytes (6). Earlier publications reported that seasons of birth are a potential risk factor for the development of MS later in life. The maximum and minimum values were confirmed to be more related to spring and autumn, respectively (7). A statistically significant association between month of birth (MOB) and MS in 1035 patients in Kuwait showed that there were 13% more MS births during December (8). An overall risk of migrant’s MS birth showed slight tendency from September through February (9). A disease progression study in a large cohort of Italian patients showed no correlation between MS and MOB (10). Data from Latin America and Portugal showed deficiency of seasonality model and doubted considering MOB as a specific risk factor for MS (11). Study of different latitudes of South America showed significant variation. Patients born to mothers who were pregnant at different Southern latitudes do not follow the seasonal pattern observed at high Northern latitudes (12). Regarding the MOB and risk of MS, vitamin D, as a promoter of some alleles, also promotes Th2 function and inhibit the proinflammatory cytokines, IL-1a, IL-2β, and TNFα (13). A study in Sweden on 459 patients with MS reported that 25-hydroxy vitamin D at birth was not associated with risk of MS (14). Study of 6649 Norwegian patients with MS showed a higher frequency of April births (15). In 307 patients with longitudinally extensive spinal cord lesions, the pathogenesis of MS was correlated with MOB, which suggested some role for environmental factors (16). Study of Portugal group on 421 patients with MS could not support the seasonality of MOB as risk factor for MS (17). The correlation between MOB and the risk of MS seems to the most important in high-risk districts, particularly in the regions with low sunlight exposure (18, 19). Study of 6649 Norwegian patients with MS confirmed increase and decrease risk of MS in spring and winter, respectively (20).
2. Objectives
While reported prevalence for Isfahan with a prevalence of 93.6 per 100000 people recommended a high risk area for MS, (21) this study aimed to assess the correlation between MOB and risk of MS within this population.
3. Patients and Methods
A total of 1484 patients with MS were studied at the Isfahan University of Medical Sciences, Isfahan, Iran. Patients’ information such as first name, surname, age, date of birth, record No, and date of neurology visiting for methylprednisolone pulse therapy were recorded in a database. For each individual patient, day, month, and year of birth were converted from Persian calendar to Gregorian calendar. As a result, date of birth was recorded in the database in two forms: Persian and Gregorian. The converted Gregorian date of birth was used for further analysis. Iran is located the Northern hemisphere, more exactly, in Eastern Northern hemisphere. Northern hemisphere seasons include spring (1 March to 31 May), summer (1 June to 31 August), autumn (1 September to 30 November), and winter (1 December to 28 Friday). The statistical analysis of database was performed using (SPSS; Version 18)
4. Results
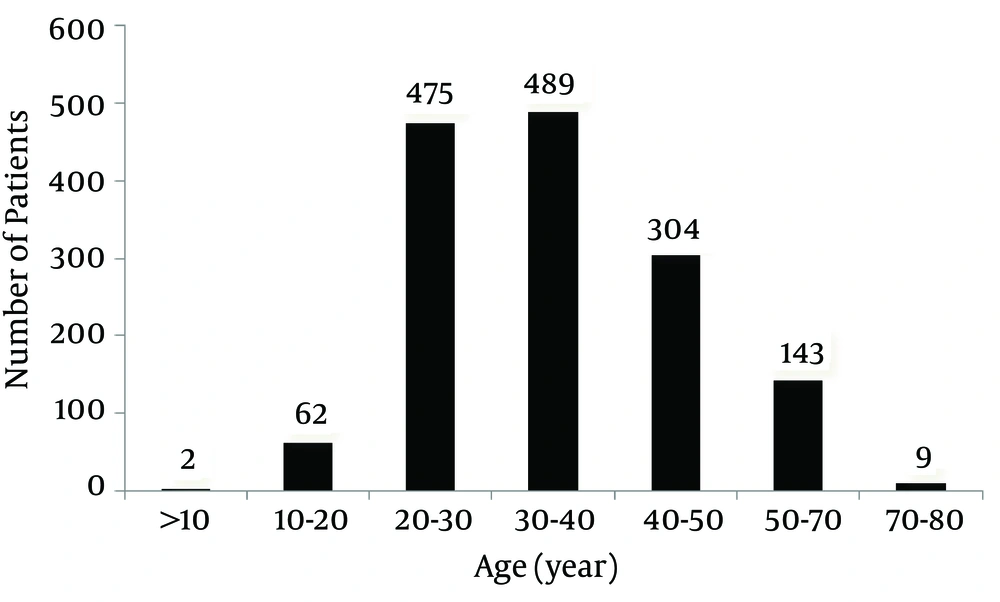
There were 1111 females (75%) and 373 males. Distribution of patients’ age was as follows: 10 to 20 years old, 4%; 20 to 30 years old, 32%; 30 to 40 years old, 33%; 40 to 50 years old, 20%; 50 to 70 years old, 10%; and 70 to 88 years old, 1%.
As shown in Figure 1, the patients’ age ranged from seven to 88 years old with 65% of patients being 20 to 40 years old.
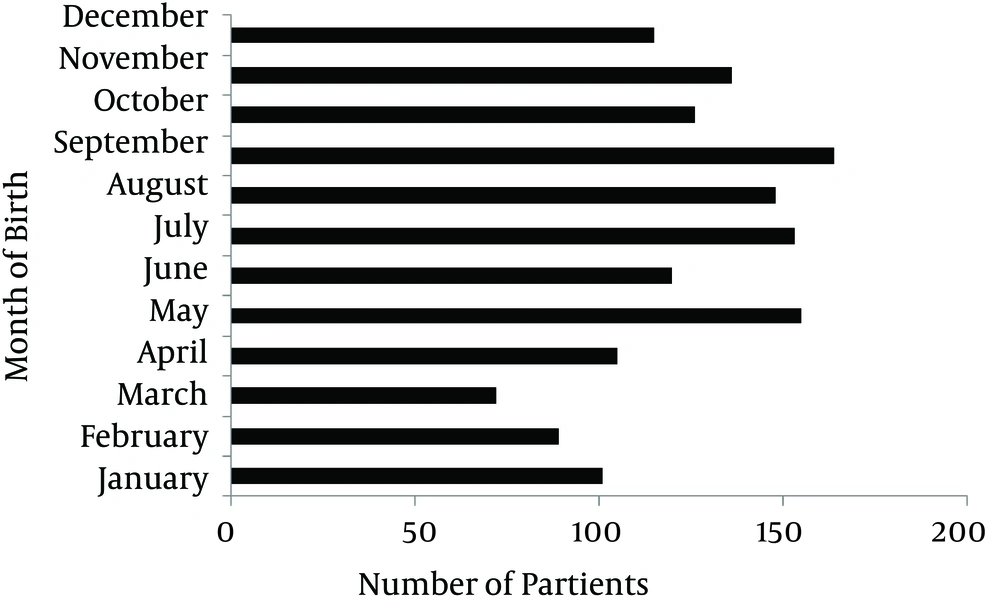
As shown in Figure 2, analysis of most frequent months of birth were consecutively September (n = 164), May (n = 155), July (n = 153), August (n = 148), November (n = 136), October (n = 126), June (n = 120), December (n = 115), April (n = 105), January (n = 101), February (n = 89), and March (n = 72). In comparison to other months, the number of patients with MS seems to be increased in April, May and September.
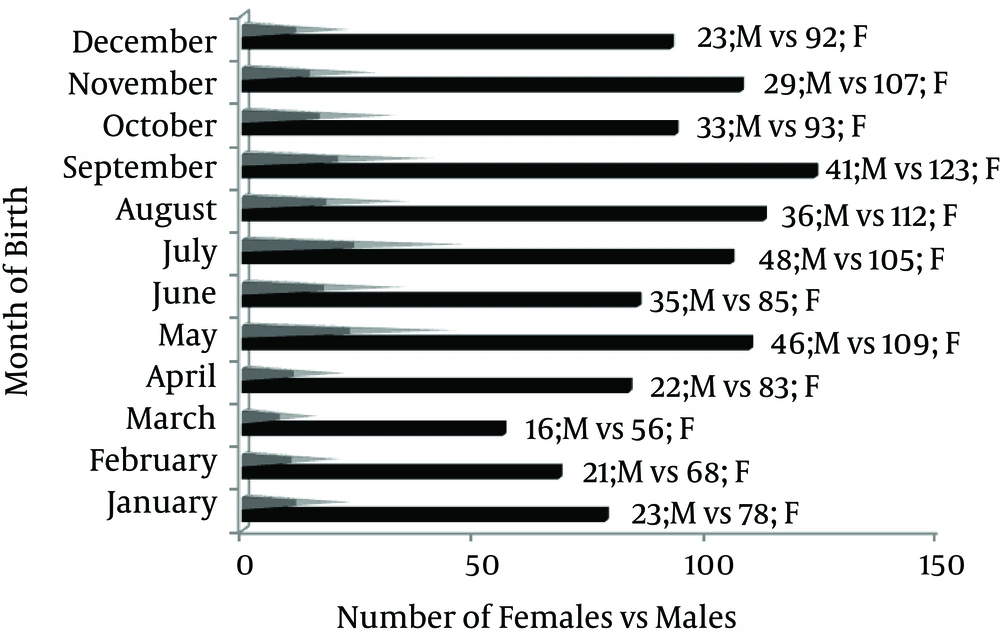
A further analysis of females (n = 1111) with a mean age of 33.8 years (range, 8-87 years) showed that the most frequent MOBs were consecutively September (n = 123), August (n = 112), May (n = 109), November (n = 107), July (n = 105), October (n = 93), December (n = 92), June (n = 85), April (n = 83), January (n = 78), February (n = 68), and March (n = 56) (Figure 3). In males (n = 373) with the mean age of 37.1 years (range, 7-88 years), the most frequent MOBs were consecutively July (n = 48), May (n = 46), September (n = 41), August (n = 36), June (n = 35), October (n = 33), November (n = 29), December (n = 23), January (n = 23), April (n = 22), February (n = 21), and March (n = 16) (Figure 3).
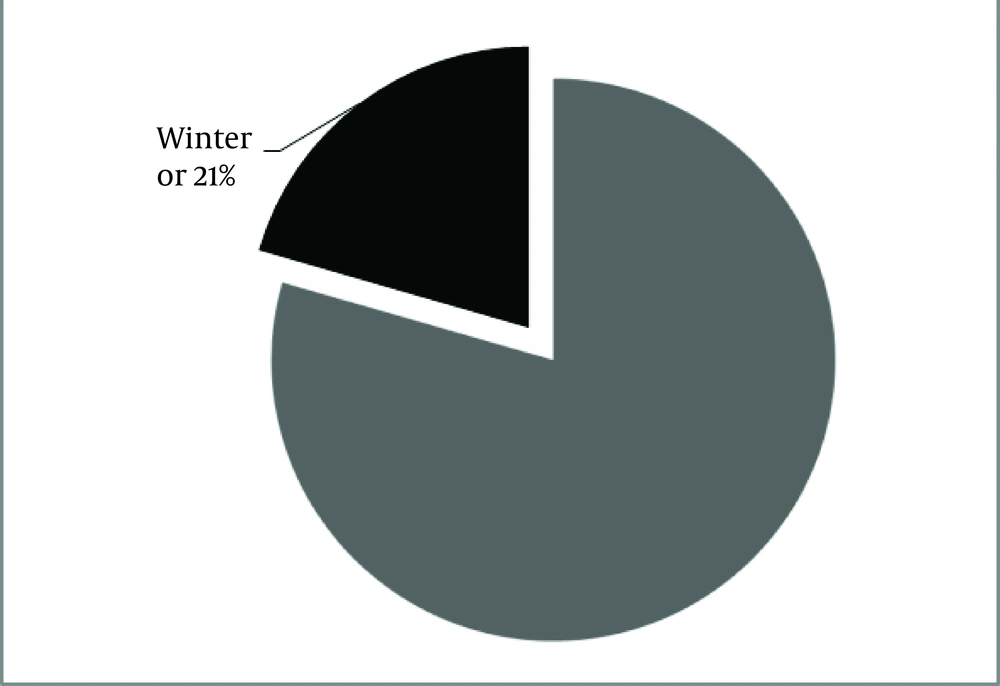
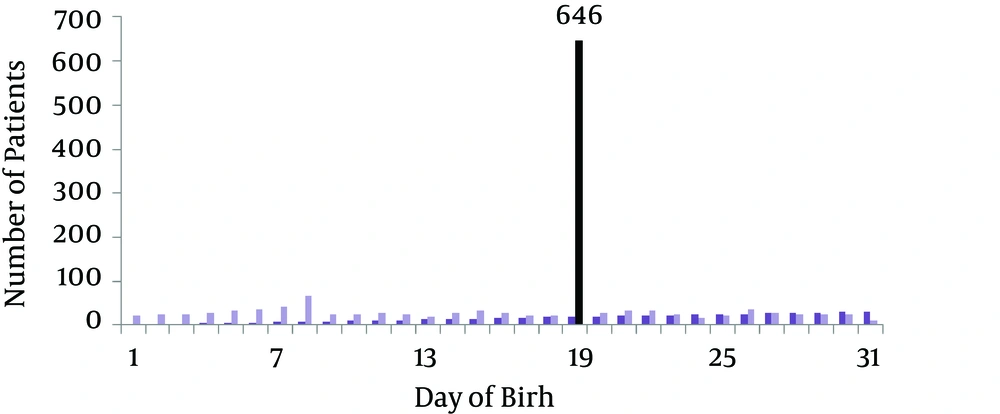
Regarding the season of birth, adjusted date of birth according to the Northern hemisphere showed that the season of birth was associated to winter in the 21% of patients (Figure 4). A further analysis of the day of birth showed that it was in the second part of the month (after the 15th day) in the 69% of patients. Of this population, date of birth was the 19th day of month in the 44% of patients (Figure 5).
5. Discussion
MS is a provocative disease distinguished by losing the myelin sheath of neurons in the central nervous system (22). Many factors such as environmental and genetic factors may play important role in developing MS (23). Susceptibility to develop MS and its association with MOB has been confirmed in some studies (10). The observed May/November birth ratio for places suggested that environmental factors are maternally mediated and influence development in the nervous or immune system, or both (24). Seasonal nutrient dissimilarity may also affect fetal growth at a cellular level (25). A MOB (May/November) effect shown in MS suggests that an unspecified environmental effect in early development can affect both MS susceptibility and phenotype (26). An excess of MS births in spring and a decrease in autumn (Northern hemisphere) also has been reported (18). Study performed by Koch reported that the season or MOB does not appear to influence disease progression (27). The possibility of maternal exposure to sunlight in increasing the development of MS later in life has been mentioned previously (5, 6).
In this study, the lowest population of patients with MS (21%) was seen in winter. The highest prevalence was started from April, with a peak in May and September. In consistency with previous reports, only 25% of populations were males. Age in 65% of patients ranged from 20 to 40 years. This young society could provide a major financial burden for patient, family, and Iranian Ministry of Health.
To explain these results, many factors could influence the high prevalence of MS in young Iranian with MS. While hereditary feature might figure for a large susceptibility, geo-epidemiologic investigations propose the important role of environment regarding the disease commencement and intonation. A published report confirms that up to 20% of all MS cases are familial (28) and a further research connects MS to the HLA-DRB1 locus, with the HLA-DR15 haplotype (29). In another study, it has been confirmed that rs10767935 and rs5030244 in WT1 modify the IFN-β-25 (OH) D association in patients with MS (30). polymorphisms in genes involved in T-helper cell differentiation are associated with risk of developing MS. Initiation of neuroinflammation in animal models of MS has been shown to be dependent on T-helper cell-derived granulocyte-macrophage colony-stimulating factor (31). Lin X showed that 45 SNPs act as cis-effect regulators on 19 MS-associated genes. Among the 45 SNPs, 15 are most likely situated in transcription factor-binding sites, and five predicted SNPs (rs3095329 of TUBB, rs9469220/rs2647046 of HLA-DQB1, rs11154801 of AHI1, and rs1062158 of NDFIP1) have corresponding target genes with significantly differential expressions in multiple cell groups, while rs7194 of HLA-DRA is predicted to be in the has-miR-6507-3p binding site (32).
Regarding the environmental variables, recent publication classified many factors such as low obedience related to pharmacotherapy management, smoking, overweightness, low concentrations of vitamins A and D, use of high salt in food, and sedentary lifestyle (33).
In addition, not only the energy of biosphere and circadian rhythms are provided by solar radiation, but also genomes may be vigorously changed by it. The skin synthesis of vitamin D, as a strong immune modulator, after exposure to solar radiation could be changed by geographic and seasonal variation (34). The hours of daily exposure to sunlight or photoperiod could be also accountable for the association with latitude. The predictable affinity for latitudinal and chronological association might demonstrate advanced rates of birth in spring months and lower rates of birth in winter months (35). Additional research on risk factors related to genetic and environmental features seems to be a significant approach in the management of MS in Iranian population.