1. Context
1.1. The Immuno-Pathophysiology of Multiple Sclerosis
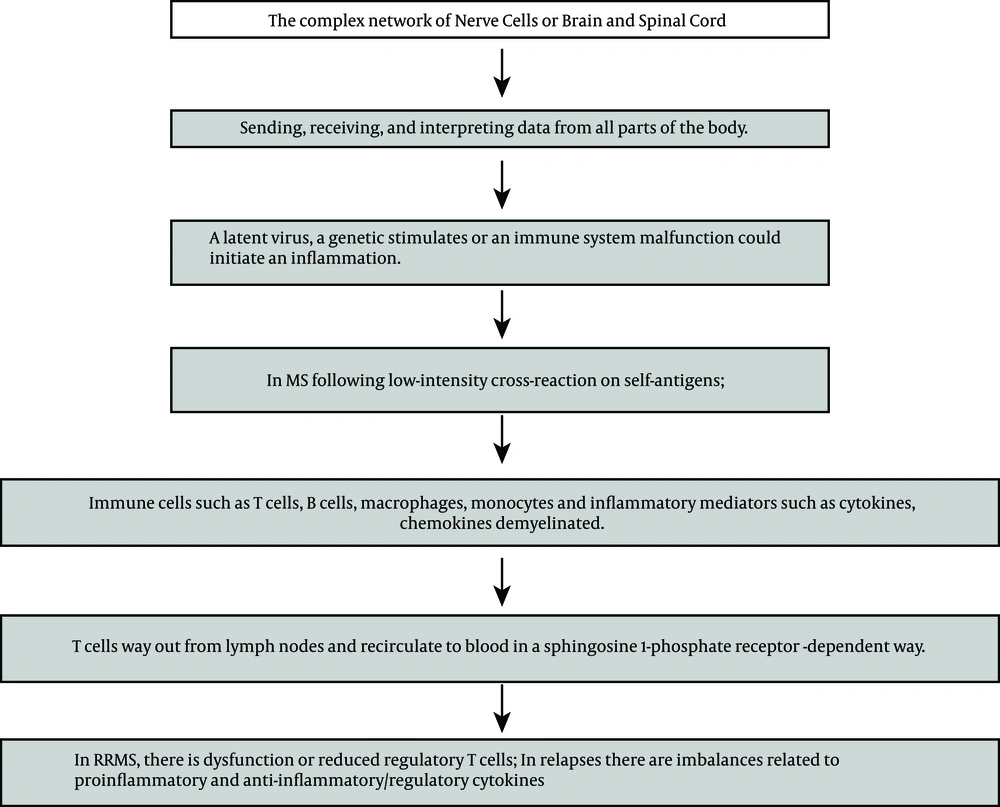
Multiple sclerosis (MS) is an immune-related disease that leads to central nervous system (CNS) irritation, demyelination and deterioration. As shown in Figure 1, in the CNS, myelin is shaped by oligodendrocytes. It surrounds and defends axons, that work to ease signal transmission. Myelin injury leads to slowing in transmission or totally congested passage, which causes the clinical manifestation of deteriorations in patients, which is called MS relapse. In this event, immune cells such as T and B lymphocytes (T and B cells), macrophages, monocytes and inflammatory mediators such as cytokines and chemokines are demyelinated as well. In fact an increased blood-brain-barrier (BBB) could be described as a significant representation of MS. Therefore expression of adhesion molecules, cytokines and metalloproteinases could cause further disruption of BBB. The key roles regarding T cells could be considered by the presence of cluster difference (CD) CD4+ T helper cells and CD8+ T cells. Activation of macrophages is a duty of T helper cell type 1 (Th1), by releasing proinflammatory cytokine Interferon gamma (IFN-γ) and tumor necrosis factor-α. The Th17 cells create key inflammatory mediators such as interleukins. Regulatory T cells (Treg) usually adjust the strength of the immune response and preserve self-tolerance by creating anti-inflammatory cytokines, such as interleukin (IL) IL-10. Furthermore, CD4+ Th2 cells produce IL-4, IL-5 and IL-13, that can change the role of macrophages (1-10). The balance between biologically active transforming growth factor (TGF)-beta1 and pro-inflammatory Tumor Necrosis Factor (TNF)-alpha, IL-1beta and IFN-γ are dysregulated during MS relapse-remission, and normal counter-regulatory mechanisms during the relapse phase are defective (11). B-cell reduction treatment could also powerfully decline disease progression in relapsing-remitting (RR) MS (12).
1.2. Pharmacotherapy Strategy for Relapsing-Remitting Multiple Sclerosis
In clinical practice, over 80% of MS is identified as RR, which is the most common subtype of the disease. However, relapses are common and represent a major feature of MS but when relapses occurs, disability is accumulated and clinical management could face a big gap among different patients with MS (1-12). In patients with MS, those that receive disease-modifying drugs (DMDs) but still experience high disease activity, require different pharmacotherapy strategies (13-18). Limitations to parenteral disease-modifying therapies (DMTs) such as interferon beta-1a, interferon beta-1b, and glatiramer acetate for the treatment of RRMS have been addressed by the approval of oral DMT-fingolimod (19). This was approved by the food and drug administration (FDA) on 21st of september, 2010. Removing lymphocytes in lymph nodes trough sphingosine-1-phosphate receptor modulator FTY720 (fingolimod or gilenya) contributes to an autoimmune reaction (1-19).
2. Evidence Acquisition
Re-evaluation in the pharmacotherapy approach for RRMS, in those experiences high disease activity despite receiving parenteral DMTs necessities additional attention. Therefore, this review aimed to provide a summary of fingolimod-therapy for MS in relation to a brief description of disease. As a result, clinical trials and review articles related to fingolimod efficacy in MS were investigated.
2.1. Survey Method
United States national library of medicine (PubMed, NLM) was searched. The key words relevant to “1) fingolimod, 2) fingolimod efficacy and safety, 3) fingolimod in multiple sclerosis, 4) fingolimod efficacy and safety in multiple sclerosis” were examined. A total of: 1) 1663 (Oct 2015 to January 1996), 2) 130 (Sep 2015 to February 2000), 3) 720 (June 2015 to June 2002), 4) 109 (June 2015 to December 2006) articles were recognized, respectively. Afterwards, articles relevant to fingolimod-therapy were selected and reviewed completely.
3. Results
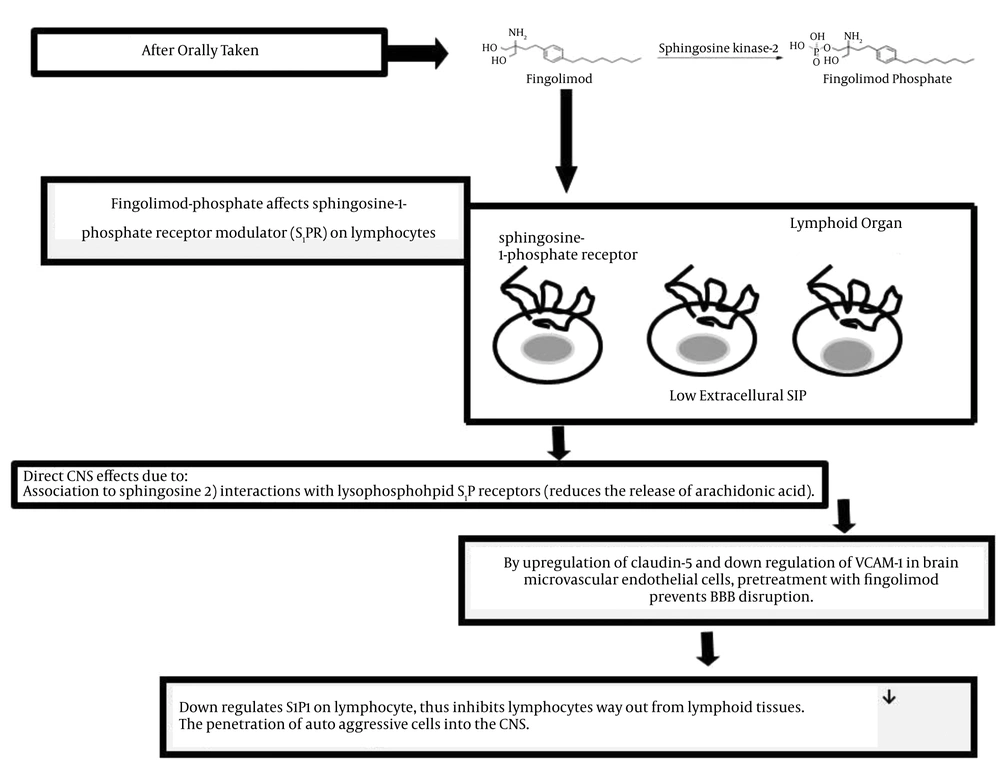
Attentive attention is needed to differentiate between relapses and steady progression, as RRMS was reported as the most common subgroup of MS (1-23). Acute or transient tingling/pins-and-needle sensations may indicate myelitis. Multiple sclerosis progression could be considered when chronic or insidious sensory change or pain syndromes occur. Unexpected faintness of both legs disturbing the ability to walk recommends MS relapse, but steady alteration in walking capability over a long period of time suggests MS progression (1, 9). Regarding the pharmacotherapy for RRMS, oral Sphingosine 1-Phosphate (SP1) receptor modulator, fingolimod, was approved for prescription since 2010 (13, 17). In year 1994, from the culture broth of the fungus Isaria sinclairii, the immunosuppressive natural product myriocin was isolated. By chemical modification of myriocin, FTY720 or fingolimod or gilenya was yielded. As shown in Figure 2, the mechanism of action related to fingolimod in MS is not completely understood. After taking the drug orally, immunological and CNS activities are initiated, and fingolimod is phosphorylated by sphingosine kinase-2. The phosphorylated form of the drug affects Sphingosine-1-phosphate Receptor Modulator (S1PR) on lymphocytes. Regarding the biosynthesis of all eicosanoids, the rate-limiting step is the phospholipase A2-mediated release of arachidonic acid from glycerol phospholipids. In response to antigen, fingolimod reduces the release of arachidonic acid (Figure 2). A previous study suggested potential therapeutic mechanism of action of fingolimod in eicosanoid-driven inflammatory disorder such as MS (16). The main inflammation inductor of fibrosis and myofibroblasts or transforming growth factor-beta is mainly accountable for extreme matrix protein creation. Fingolimod provoked myofibroblasts differentiation comparable with that of transforming growth factor-beta (21). Studies have shown that fingolimod acts at sphingosine-1-phosphate receptors on lymphocytes and endothelium, so preventing the passage of T- and B cells from secondary lymphoid organs into the blood and their movement to inflamed tissues (22, 23).
Annualized reduction in deterioration frequency and safety summary with fingolimod 0.5 mg, 1.25 mg or placebo once daily for one year, was reported in those who received interferon-β or glatiramer acetate but discontinued previous disease-modifying therapy due to inadequate beneficial events (17). A comparative study of 249 versus 257 patients (groups included fingolimod versus placebo, respectively), confirmed 48% reduction in the annualized relapse rate. Regarding three and six months disability progression, fingolimod caused 34% and 45% reduction versus placebo. Fingolimod reduced the number of new or newly enlarged T2 lesions by 69% relative to the placebo (6). An average fingolimod prescription time of 8.6 months, in patients with very active MS, before the age of 18 years, exhibited a respectable safety and effectiveness profile (18).
Another study related to 227 patients that used fingolimod pharmacotherapy for MS showed that the number of gadolinium-enhanced lesions and relapse rates continued to be low. Adverse events were 1) nasopharyngitis dyspnea 2) headache 3) diarrhea, and 4) nausea. Alanine aminotransferase levels were significantly increased in 10% to 12% of the studied population. Posterior reversible encephalopathy syndrome occurred in one patient with 0.5 mg fingolimod. An early decrease in the heart rate and a modest decline in the forced expiratory capacity in one second were also reported (21).
Regarding the conversion from interferon-β1a to fingolimod, a study of 772 patients confirmed a sustained outcome of long-term fingolimod therapy in keeping a low rate of disease progression (24).
Recently, a case study reported that after twenty days of fingolimod pharmacotherapy for a 46-year-old lady, dysarthria and lower limb weakness appeared. brain magnetic resonance imaging (MRI) showed more than 20 millimeters gadolinium (GD) enhanced lesions in periventricular white matter, juxta-cortical white matter and cerebellum (24). Studies also suggested that pharmacotherapy based on fingolimod has been shown to be superior to IFN - β (1-26).
4. Conclusions
Multiple sclerosis, the long-term aggressive demyelinating disease of the CNS, which could be verified by an extremely variable clinical course, is associated with the most common relapsing-remitting form, in over 80% of its population (27). In the year 1992, Yoshimoto Pharmaceuticals, manufactured an oral immunomodulator drug, FT720, fingolimod or gilenya (13). A previous study related to fingolimod pharmacotherapy trial for MS indicated a reduction in the rate of relapses by over half (18-30).
Due to fingolimod association with sphingosine, and its interactions with lysophospholipid S1P receptors, the drug has a direct CNS effect. The rapid onset of therapeutic effects, which have been reported 40 days after disease onset, might be related to localization of the drug within the CNS (31, 32).
By upregulation of claudin - 5 and down regulation of vascular cell adhesion molecule (VCAM - 1) in brain microvascular endothelial cells, pretreatment with fingolimod prevents BBB disruption. Therefore, in both events related to MS patients with; 1) relapsing remitting and 2) secondary progressive disease, it has been reported to be beneficial (33). Evaluation of cerebral spinal fluid (CSF) levels recommended the utility of neurofilaments as a measurable biomarker in MS and a valuable outcome of fingolimod (34).
The adverse effects in those that used fingolimod-therapy for MS could be 1) bradycardia 2) relapse 3) basal-cell carcinoma 4) macular edema 5) cancer (skin) 6) nasopharyngitis dyspnea 7) headache 8) diarrhea 9) nausea and 10) laboratory abnormalities (such as increase in alanine aminotransferase levels). The most common side effects have been reported as 1) head cold 2) headache and 3) fatigue. Death due to herpes and zoster infections was reported in two cases (27, 30-35). However, a study related to the prescription of 0.5 mg fingolimod, showed a low frequency of macular edema in MS patients but in those with a history of uveitis, it was suggested that it could increase the risk of macular edema (36). The drug seems to be associated with an increased risk of fetal malformations (37).
Finally, in order to recognize for a sensitive well-balanced pharmacotherapy strategy, in addition to considering objects such as great efficiency and simplicity of administration, aggressive outcomes such as cardiotoxicity, risk of infection and cancer should always be in mind.
| Fingolimod (Gilenya or FTY720) |
|---|
| Mechanism of Action: Sphingosine 1-phosphate receptor modulator |
| By Prescription Only |
| Route of administration: Only oral |
| Available as: Tab 0.5 and 1 mg |
| Approved: 21st of Sep 2010 |
| Was Available in Canada: 10th of March 2011 (Pharmacies) |
| European Union: 17th of March 2011 |
| Serious Adverse Effects:1) Bradycardia 2) Relapse 3) Basal-cell carcinoma 4) Macular edema 5) Cancer 5) Fetal malformations 6) Laboratory abnormalities. The most common side effects have been reported as head cold, headache and fatigue. Death due to herpes and zoster infections was reported in two cases correspondingly |
| Reported Adverse Effects: 1) Nasopharyngitis dyspnea 2) Headache 3) Diarrhea 4) Nausea 5) Increase in alanine aminotransferase levels 6) An early decrease in heart rate 7) A modest decline in the forced expiratory capacity in one second 8) Posterior reversible encephalopathy syndrome in one case 9) Dysarthria and lower limb weakness 10) Bradycardia 11) Atrioventricular block 12) One cardiovascular- linked death |