1. Context
The human lesser superficial petrosal nerve [or nervus petrosus minor (1)], also called simply the lesser petrosal nerve (LPN), is a branch of the glossopharyngeal nerve (IX) with contributions from the facial (VII) (2-5) and vagus (X) nerves (2). It is named in contrast to the more “famous” greater superficial petrosal nerve (GSPN), branch of the VII. Compared to the latter, the gross and clinical anatomy of the LPN is often underestimated.
The LPN can be considered to arise from the tympanic branch of the IX, which passes across the plexus of the tympanic nerve and then regroups to cross the floor of the middle cranial fossa (6). The formation and course of the LPN varies significantly. This variability, as well as the small size of the LPN, could provide a possible explanation for the current lack of information regarding the course of the LPN, which is complicated and difficult to follow. This nerve could be also mistaken for the (better studied) GSPN during operations in the middle cranial fossa area (2). The purpose of this article was to review the literature regarding the clinical anatomy of the LPN.
2. Evidence Acquisition
The existing literature regarding this nerve was critically reviewed, with emphasis on its clinical significance. Specifically, a search was performed in PubMed for the term “lesser petrosal nerve,” which retrieved 23 results. Most of these articles, as well as relevant anatomy books, were used for this review. The available data are relatively restricted and include primarily human and some animal studies. Human studies are mainly cadaveric studies, although some clinical, imaging, and histological data are also available.
3. Results
3.1. Anatomy
3.1.1. Course of the Preganglionic Parasympathetic Fibers From the IX
The preganglionic parasympathetic fibers of the IX originate from the parasympathetic inferior salivary nucleus (7, 8) of the medulla oblongata and pass through the superior and inferior ganglia of the IX (9). The tympanic branch of the IX [or tympanic nerve (10), or tympanic nerve of Jacobson (9), or Jacobson’s nerve (10, 11)] carries these fibers (7), and it branches from the IX, either within or immediately outside the jugular foramen (10), just below the IX ganglion (8), on the medial side of the jugular bulb (2). It reenters the temporal bone through a small foramen on the ridge of bone separating the jugular foramen from the carotid canal and ascends through the inferior tympanic canaliculus (a small bony canal). It then reaches the medial wall of the tympanic cavity and divides into branches (2, 10). In the middle ear, on the promontory, the branching tympanic nerve mixes with sympathetic and parasympathetic fibers from the VII (8) and participates in the formation of the tympanic plexus [located at the tympanic cavity (9)] (10).
3.1.2. LPN Formation
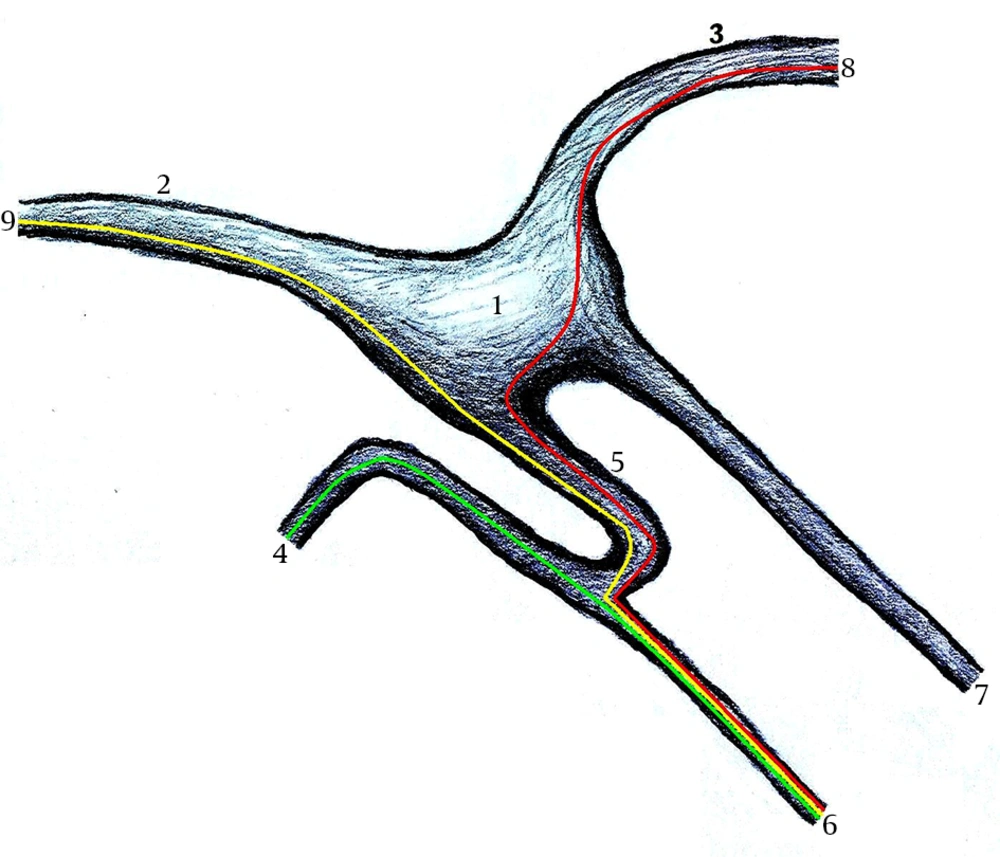
The LPN (considered by anatomists as a branch of the tympanic plexus) originates at the region of the geniculate ganglion (11) (Figure 1) and carries mainly preganglionic parasympathetic fibers from the tympanic plexus (7, 10, 12) to the parotid gland. Although its largest source of fibers is from the tympanic branch of the IX (2), the LPN is actually the result of a composition of fibers contributed by three different nerves: 1) the tympanic branch of the IX (2, 9-11), 2) the nervus intermedius of the VII (2, 11), and 3) the auricular branch of the X (Arnold’s nerve) (2).
1, Geniculate ganglion; 2, Facial nerve (tympanic segment); 3, Facial nerve (labyrinthic segment); 4, Tympanic branch of the glossopharyngeal nerve (Jacobson’s nerve); 5, The communicating branch; 6, Lesser petrosal nerve; 7, Greater superficial petrosal nerve; 8, Nervus intermedius; 9, Auricular branch of the vagus nerve (Arnold’s nerve).
1) The tympanic branch of the IX, after joining the tympanic plexus, crosses the promontory surface in the medial wall of the tympanic cavity and provides a branch to the LPN. The latter emerges posterior to the tensor tympani muscle (on the middle cranial fossa). 2) The fibers to the LPN from the nervus intermedius leave the VII, usually in the geniculate ganglion area. 3) A branch arising from the auricular branch of the X is the third source of the LPN fibers. This contributor to the LPN branch ascends along the anterior edge of the VII mastoid segment and then penetrates the stapedial muscle. A portion of its fibers continues through the muscle (anteriorly) and through the sheath of the VII (tympanic segment) and then travels on the surface of this nerve, but remaining separate from it. These fibers pass forward before they join the LPN (2).
The nervus intermedius fibers, as well as the auricular branch of the X, usually separate from the (tympanic segment of the) VII in the geniculate ganglion area as a discrete bundle (the communicating branch, evolved from the embryonic communication between the geniculate and IX ganglia), which joins the fibers from the tympanic branch of the IX to form the LPN (Figure 1). Two communicating branches rarely arise from the facial sheath (from the nervus intermedius and the auricular branch of the X) (2).
Kakizawa et al. (2), in their highly detailed study of the LPN, reported three types of this nerve, based on the confluence site of the three different bundles contributing to its formation. In type A, a communicating branch, consisting of fibers from the auricular branch of the X and the nervus intermedius, merges with the tympanic branch to form the LPN. In type B, two communicating branches arise from the VII and merge with the tympanic branch at different points. In type C, one communicating branch joins with the tympanic branch after traveling with a meningeal branch of the mandibular nerve (V3) (2).
3.1.3. The LPN Course
The LPN leaves the middle ear and enters the middle cranial fossa through a small opening on the anterior surface of the petrous part of the temporal bone [the canal of the LPN (1) or the canaliculus innominatus for the LPN (13)] just lateral and inferior to the opening for the GSPN. The LPN runs under the dura (between the petrous branch of the middle meningeal artery medially and the superior tympanic artery laterally) (14), at its smaller (compared to the GSPN) groove (12). The course of the LPN and GSPN usually diverges (11.6º) in the area medial to the geniculate ganglion (2). The GSPN can also run parallel to the LPN (15). A part of the LPN is often exposed on the floor of the middle cranial fossa (75%), but it can also be totally covered by thin bone (25%) (2). In its course along the middle fossa floor, the LPN is located above the tensor tympani muscle (2). The mean length of the LPN is 15 mm (from the geniculate ganglion to the foramen, where it exits the middle cranial fossa) (2).
The LPN course across the floor of the middle cranial fossa, anterior to the GSPN (Figure 1), passes medially and descends to reach the foramen ovale of the sphenoid bone, where it is widely accepted to pass through (together with the V3, accessory meningeal artery and emissary veins) (2, 8, 10, 16), because the otic ganglion is located medial to the V3 just below this foramen (2). However, no LPN examined in the study of Kakizawa et al. (2) passed through the full length of the foramen ovale. Most LPNs (70%) passed through the small canaliculus innominatus (located posterior to the foramen ovale and spinosum in the sphenoid bone) to exit the middle skull base (2). Alternatively, the LPN can pass through the sphenopetrosal fissure (1, 2), the foramen spinosum (2), or the petrosal foramen (1). Kakizawa et al. (2) reported that two of the LPNs that passed through the foramen spinosum passed through its full length, whereas one penetrated its osseous wall, to pass through the bone, and penetrated the foramen ovale wall to finally exit the skull base.
High-resolution computed tomography (CT) axial sections usually show the canaliculus innominatus, whereas this visualization is much more difficult in coronal sections. The importance of defining this structure is to avoid overlooking lesions (tumor or vascular) developing in its vicinity (17). Ginsberg et al. (13) examined 123 high-resolution CT images of the temporal bone and confirmed the existence of variations of the foramen ovale and canaliculus innominatus.
The LPN carries the parasympathetic fibers from the superior and inferior salivary nuclei through the fibers coming from the nervus intermedius, X, and IX. Sympathetic fibers for the parotid gland arise from the plexus of the middle meningeal artery, passing through the otic ganglion. Interestingly, sympathetic fibers may also reach the LPN in the floor of the middle cranial fossa through a communication with the meningeal branch of the V3 (observed in approximately one third of LPNs) before reaching the otic ganglion (2). Table 1 summarizes the contribution of different nerve fibers to the LPN.
| Cranial Nerve | Branch |
|---|---|
| IX | Tympanic branch |
| VII | Nervus intermedius |
| X | Auricular branch |
| V | Meningeal branch of the mandibular nerve (V3) |
Abbreviations: IX, glossopharyngeal nerve; LPN, lesser petrosal nerve; VII, facial nerve; V3, mandibular nerve; X, vagus nerve.
The presence of ganglion cells within the middle ear (“ectopic” ganglion cells) is also a common finding, and is considered to represent an anatomic variant. These cells are observed most often in the GSPN (38.7%), the LPN (12.4%), and the promontory (11.4%) (18). In rats, the tympanic nerve originates from the IX, enters the tympanic cavity, and crosses the promontory to pass the tensor tympani muscle. It then continues intracranially to the otic ganglion as the LPN, after its intersection with the GSPN (19).
3.1.4. The Course of the Postganglionic Parasympathetic Fibers From the IX
In the infratemporal fossa, the LPN reaches the otic ganglion, which hangs off the nerve to the tensor tympani (8). The preganglionic parasympathetic fibers synapse with the cell bodies of the postganglionic parasympathetic fibers in the otic ganglion located on the V3 (medial side), around the origin of the nerve to the medial pterygoid (10). The sympathetic fibers that join the nerve from the middle meningeal artery pass right through the otic ganglion (8). The postganglionic parasympathetic secretomotor fibers from the otic ganglion pass to the parotid salivary gland through the auriculotemporal nerve (originating from the V3), which lies in contact with the deep surface of the gland (7, 8, 10, 12).
The parasympathetic fibers leave the otic ganglion in the LPN, traveling on the anteromedial inner surface of the tympanic bulla to reach the GSPN, and then they deviate to the internal carotid artery wall via the deep petrosal nerve (20). In rats, these fibers distribute to the cerebral blood vessels (21).
3.2. Clinical Conditions and Neurosurgery
Combined damage to the GSPN and LPN is associated with paradoxical phenomena including face hyperemia, abundant salivation from the parotid gland (both following atropine administration), lacrimation, and mucus secretion from the nose (during eating). These phenomena are explained by the development of an ephaptic (“false”) relation between the damaged nerves (22). Levin (23) observed the appearance of the crocodile tears syndrome, mucus secretion, and severe hyperemia of the paretic hemiface during profuse salivation as a response to atropine (salivary atropine paradox) in a patient 2.5 years after a cranial injury. The patient had a closed head trauma with paresis of the VII, a petrosus apex fracture with a bony bridge over the nerve, and a subdural hematoma for which he had undergone an operation. Other patients with similar trauma who had no operation demonstrated the same group of symptoms. The author suggested that the crocodile tears syndrome is due to an ephaptic union between the central portion of the damaged LPN and the peripheral portion of the GSPN (23).
Sectioning of the GSPN and LPN is considered a treatment option in patients with migrainous neuralgia, where pharmaceutical treatment fails (or severe side effects are observed) and where trigeminal rhizotomy is ineffective (24).
Imauchi et al. (25) reported a middle-aged male with palsy of the left abducens nerve due to secondary foci of metastatic esophageal carcinoma to Rouviere’s node, infiltrating the petrous portion of the ipsilateral temporal bone, and to cancer invasion of the GSPN, LPN, and tensor tympani muscle (revealed with post-mortem temporal bone histology). Their findings support metastasis from the upper part of the esophagus to Rouviere’s node and direct invasion of the skull base (middle fossa) and the temporal bone, and further infiltration of the middle ear via perineural invasion (25).
From a neurosurgical point of view, an understanding of the relationships of the LPN will reduce the probability of its confusion with the GSPN when operating in the middle cranial fossa. Mistaking the LPN for the GSPN could lead to inappropriate direction of the approach to the VII distal to the geniculate ganglion or to the tympanic cavity. This mistake is most probable if the floor dura of the middle cranial fossa is being elevated from an anterior to a posterior position, thus exposing the LPN before the GSPN. This error can also happen if the GSPN is not visible due to potential lifting en bloc with the dura (2).
Regarding the LPN components, Kakizawa et al. (2) reported that the auricular branch of the X has a different color and thickness than the bundle from the nervus intermedius when observed during surgery (under the microscope). Preservation of the anterior surface of the sheath of the VII (tympanic segment) or of the geniculate ganglion, where the fibers constituting the communicating branch emerge (2), is of paramount importance.
Finally, regarding the foramen ovale, knowledge of its anatomy is important for all neurosurgical procedures involving the trigeminal nerve and administration of anesthesia in the V3. Interestingly, the percutaneous biopsy of the cavernous sinus is also performed through the foramen ovale (16). Box 1 summarizes the human skull foramina where the LPN can be found.
| Foramen |
|---|
| Canaliculus innominatus |
| Foramen ovale |
| Sphenopetrosal fissure |
| Foramen spinosum |
| Petrosal foramen |
Abbreviation: LPN, lesser petrosal nerve.
4. Conclusions
The LPN is anatomically one of the most complicated nerves in humans. Quite thin and small, it receives contributing fibers from up to four different cranial nerves, and it passes from the middle ear to the middle cranial fossa through a small opening of the petrous part of the temporal bone. The LPN follows a variable and complicated course to serve primarily the preganglionic part of the parasympathetic innervation of the parotid gland. Its clinical significance results mainly from its close relation to the GSPN, with the crocodile tears syndrome being a representative example. During neurosurgical procedures, particular care is needed to avoid mistaking the LPN for the GSPN. To avoid this error, the dura matter of the middle fossa floor should be elevated from the posterior to the anterior.