1. Context
A significant proportion of global burden of disease is caused by mental, neurological, and substance use disorders (1). A study conducted in 2015 reported the worldwide prevalence of mental disorders as 13.4%, which is high (2). In some studies, low prevalence of psychiatric disorders was reported in the elderly, high prevalence of mood and anxiety disorders was reported in females, and substance use and several personality disorders were reported in males (3, 4).
Studies showed that mental disorders are a risk factor for blood borne infections (5-7). Borna disease virus (BDV) is one of the infections considered by some studies as a risk factor that might play a role in the development of neurological and psychiatric disorders. However, there is no consensus on whether BDV can infect human and cause neuropsychiatric disorders (8). BDV is a negative strand and negative polarity, non-segmented RNA virus belonging to order Mononegavirales that replicates in the cell nucleus (9, 10). BDV mainly infects the cortex and hippocampus neurons of limbic system and thus, may affect cognition and behavior (11).
Bechterand et al., detected BDV immunoglobulin G (IgG) in cerebrospinal fluid (CSF) specimen of patients with mood disorder and confirmed the role of BDV infection in the etiology of psychiatric disorders (12). In a study using real-time reverse transcriptase polymerase chain reaction (RRT-PCR) to detect BDV p24RNA in patients with mood disorder, the association between BDV infection and mood and other psychiatric disorders was reported (13). In another study by Li et al., using western blot (WB) assay and RT-PCR, BDV was detected in 6 patients with encephalitis, but not in the ones with other neurological disorders (14). No association was observed between BDV infection and psychiatric disorders including depressive, bipolar, and schizophrenia in a study using RRT-PCR and indirect immunofluorescence antibody (IFA) methods (15). Tsuji et al. compared patients with psychiatric disorders with a control group using RT-PCR and WB assay, but did not confirm the association between BDV infection and mood and other psychiatric disorders (16). In another study conducted in Iran, high prevalence of subclinical BDV infections and the association between BDV infection and mood disorders such as bipolar and major depressive disorders (MDD) was approved (17). The study by Horing et al. rejected the role of BDV infection in pathogenesis of psychiatric disorders such as bipolar, major depressive, and schizophrenia (18).
Moreover, the evidence indicated a possible association between BDV infection and mood disorders, but the exact association was not clear and many questions about this association remained unanswered.
2. Objectives
3. Methods:
3.1. Study Protocol
The current study was conducted using the preferred reporting items for systematic reviews and meta-analyses (PRISMA) guidelines (20). To avoid bias, all steps of the study were performed by 2 researchers independently. A third author reviewed any disagreement between the 2 researchers.
3.2. Data Sources
To obtain the corresponding evidence, the following databases were searched: PubMed, Scopus, ScienceDirect, Web of Science, Embase, Cochrane Library, PsycINFO, and Google Scholar search engine as well as the reference list of the articles without time limit until 15 January 2017. The search was developed using the following keywords: “Borna Disease Virus”[MeSH], “Mood Disorders”[MeSH], “Mood Disorders”[MeSH], “Depression”[MeSH], “Depressive Disorder"[MeSH], “Bipolar Disorder”[MeSH], “Depressive Disorder, Major”[MeSH], “Infection”[MeSH], and “Mental Disorders”[MeSH].
3.3. Inclusion and Exclusion Criteria
The inclusion criteria were observational epidemiological studies (i e, case-control, cohort, and cross sectional) in English on human populations. Studies with insufficient data, irrelevant to the topic, without the outcome of mood disorders based on exposure to BDV along with a control group of healthy people for comparison, letter to editors, presentations, review articles, and case reports were excluded.
3.4. Study Selection and Quality Assessment
The primary screening was conducted to select relevant articles by screening title and abstract of the articles, and then the full text of the remaining articles were reviewed for eligibility. Finally, articles were assessed using the modified Newcastle Ottawa Scale (NOS) for non randomized studies and articles that got a minimum score of 7 were selected (22).
3.5. Data Extraction
The data of the selected articles including the name of the first author, country, year and design of study, name of journal, characteristics of samples (e g, sample size, gender, and mean age), diagnostic method, diagnostic criteria, odds ratio (OR), 95% confidence interval (CI), and number of BDV positive subjects in the case and control groups were extracted and recorded in an Excel file.
3.6. Data Analysis
The data were analyzed using the Comprehensive Meta-Analysis ver. 2 software. OR was used with 95% CIs as a selective association index and forest plot was used to present combined result and individual studies separately. To assess the heterogeneity of the studies, Cochran’s Q test and I2 statistic were used (I2 index lower than 25%, 25% - 75%, and higher than 75% was considered as low, moderate, and high heterogeneity, respectively) (23). Due to the heterogeneity of the studies, the random effect model was used to combine the study data. Subgroup analysis was performed according to diagnostic method and bipolar and unipolar disorders. The sensitivity analysis was employed to investigate the stability and reliability of data by deleting every single study from meta-analysis. To assess publication bias, the Egger and Begg's tests were used. The significance level was considered < 0.05.
4. Results
4.1. Search Results
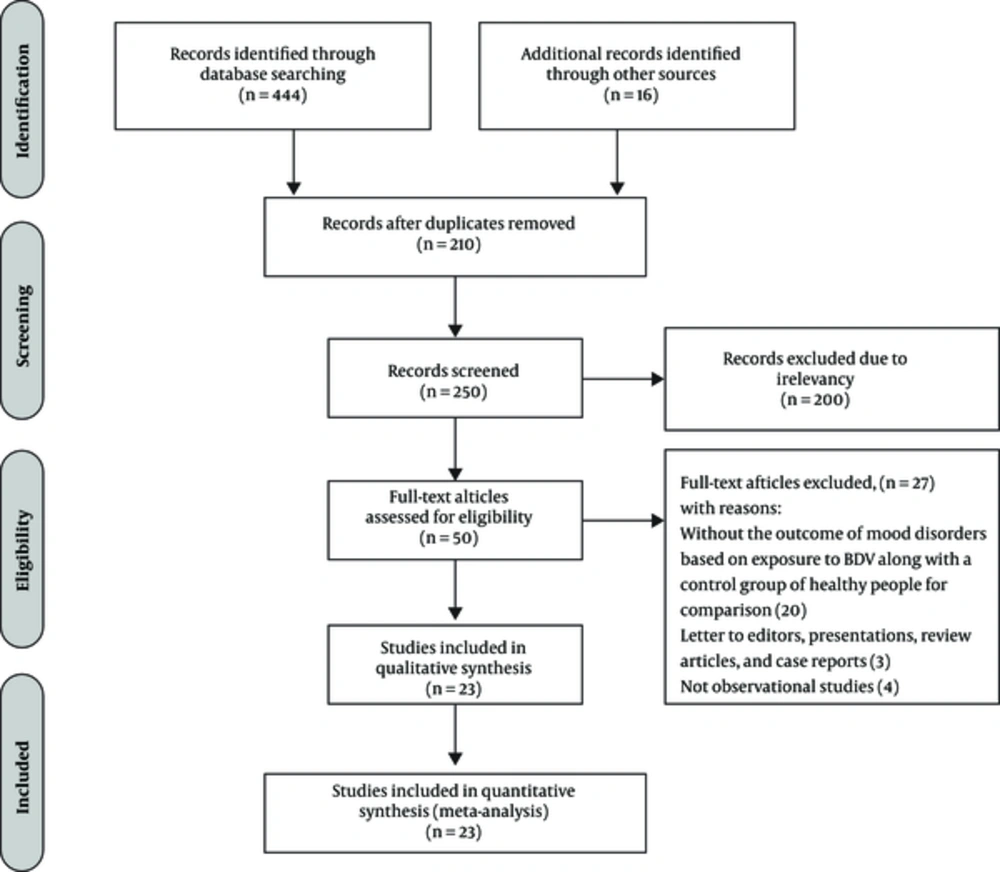
In the initial systematic search, 460 publications were found; 210 duplicates were excluded. After screening titles and abstracts of the 250 retained studies, 200 irrelevant ones were excluded. Then, the full texts of the remaining articles were studied and 27 articles were excluded (Figure 1).
4.2. Characteristics of the included studies
Finally, 23 eligible studies consisting of 3628 cases and 5810 controls entered the final analysis (Tables 1 and 2).
| Subtype | Study (N) | OR (95%CI) | P Value | Heterogeneity | |
|---|---|---|---|---|---|
| I2 | P Value | ||||
| Unipolar disorder | 10 | 1.162 (0.765 - 1.765) | 0.481 | 36.2 | 0.119 |
| Bipolar disorder | 7 | 1.996 (1.293 - 3.080) | 0.002 | 0 | 0.856 |
Abbreviations: confidence interval (CI); number (N); odds ratio (OR).
4.3. The Association Between BDV and Mood Disorders
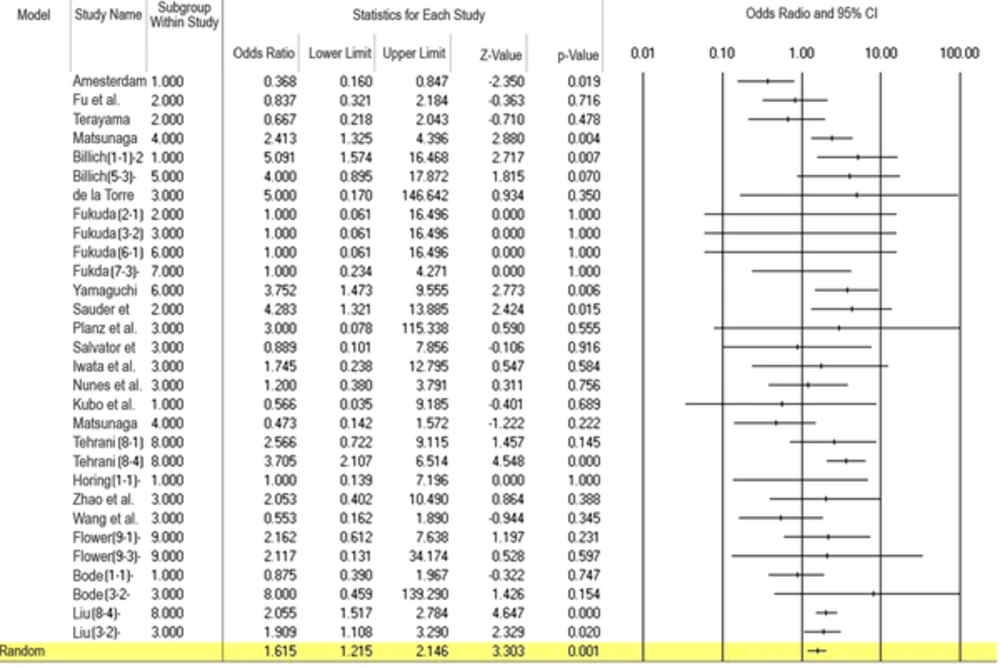
The heterogeneity rate in the current study was moderate (I2 = 44.43%, P = 0.005). Meta-analysis of 23 studies showed that BDV infection was associated with a significantly increased risk of mood disorders (OR = 1.615; 95%CI: 1.215 - 2.15, P = 0.001) (Figure 2).
4.4. Subgroup Analysis Based on Diagnostic Methods
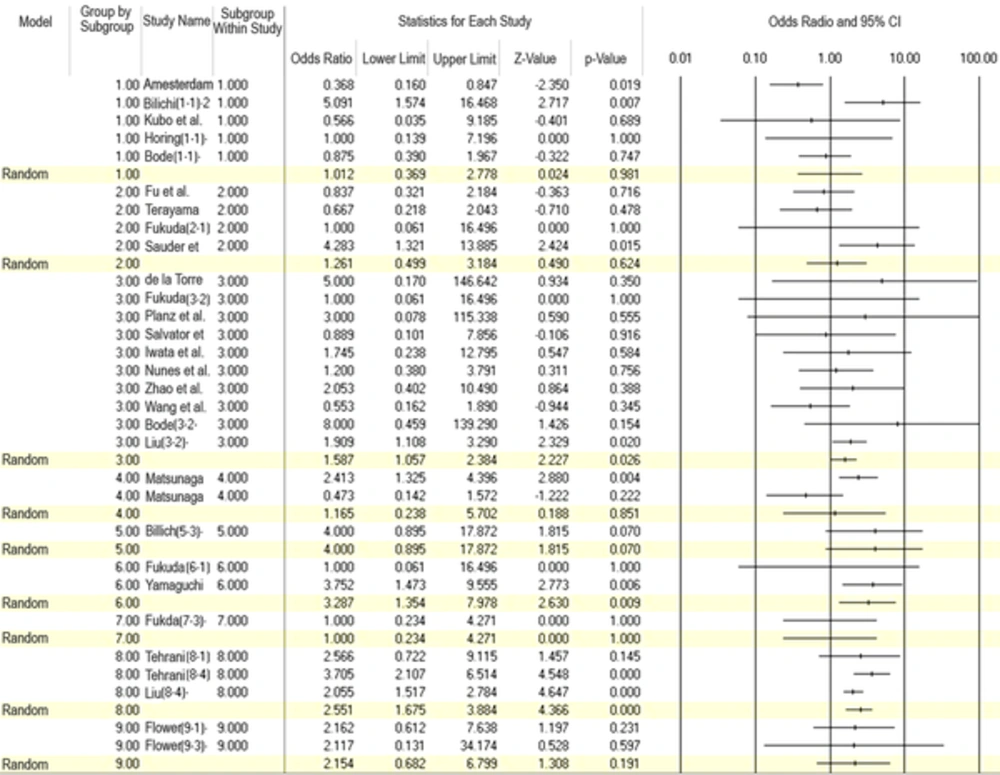
Subgroup analysis used according to diagnostic method indicated that OR was 1.01 (95%CI: 0.37 - 2.8) for IFA, 1.26 (95%CI: 0.5 - 3.18) for WB, 1.58 (95%CI: 1.06 - 2.38) for RT-PCR, 1.16 (95%CI: 0.24 - 5.70) for radioligand assay (RLA), 2.55 (95%CI: 1.67 - 3.88) for enzyme immunoassay (EIA), 2.15 (95%CI: 0.68 - 6.80) for enzyme-linked immunosorbent assay (ELISA), 3.29 (95%CI: 1.35 - 7.98) for electrochemiluminescence immunoassay (ECLIA), 4.00 (95%CI: 0.89 - 17.87) for peptide array, and 1.00 (95%CI: 0.23 - 4.27) for proliferation assay (Figure 3).
4.5. Subgroup Analysis Based on Patients with Bipolar/Unipolar Depressive Disorder
In subgroup analysis of patients with bipolar/ unipolar depressive disorder, OR was 1.99 (95%CI: 1.29 - 3.08, P = 0.002) and 1.16 (95%CI: 0.76 - 1.76, P = 0.481), respectively (Table 2).
4.6. Publication Bias and Sensitivity Analysis
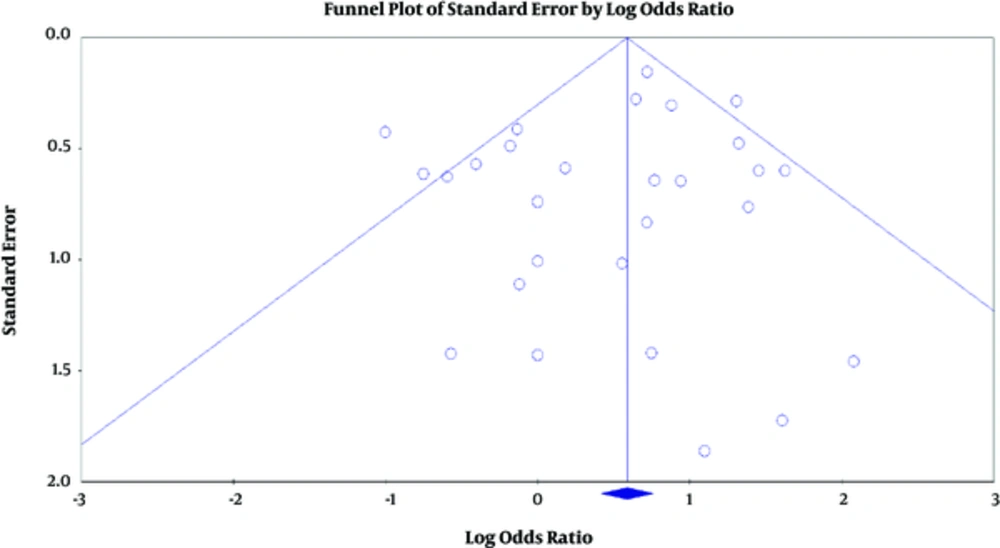
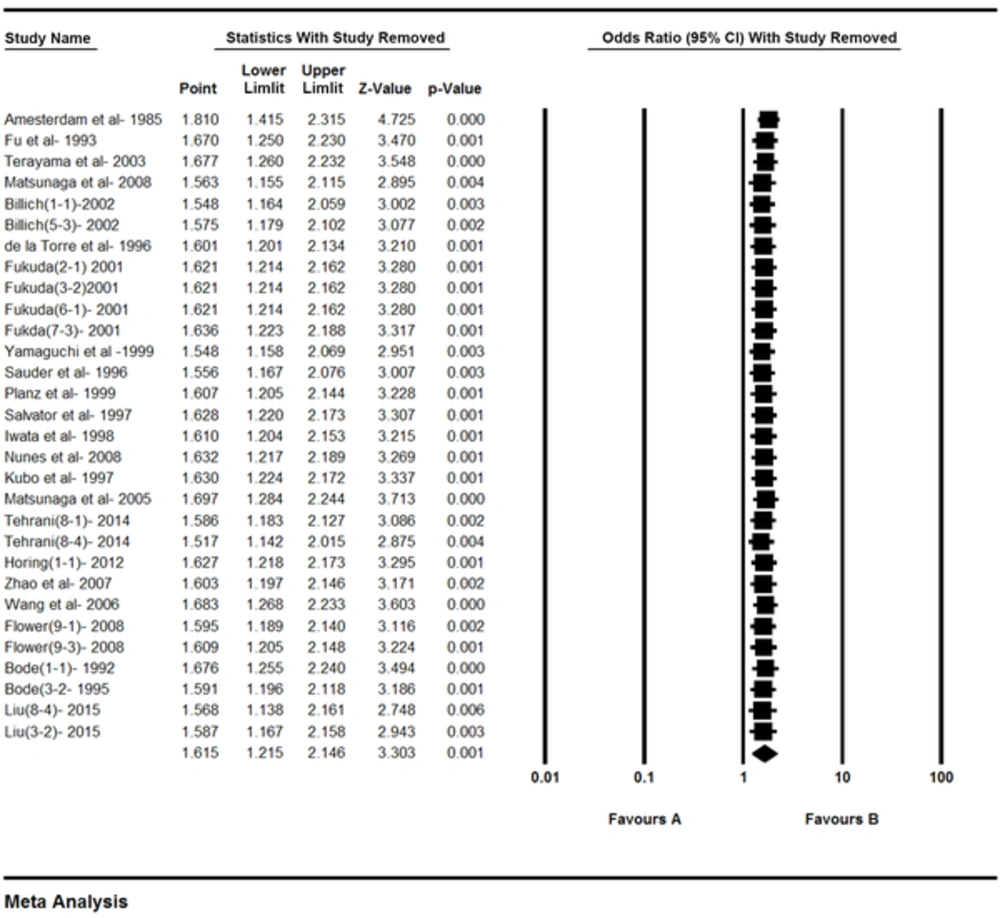
P values estimated for the publication bias in the Egger and Begg tests were 0.31 and 0.96 respectively, and the difference between them was not significant (Figure 4). In the sensitivity analysis, the impact of each study on the combined OR was determined by repeating the meta-analysis after removing 1 study, and the relationship was significant (Figure 5).
5. Discussion
In the current study, a significant association was noted between BDV infection and mood disorders by combining the results of 23 studies. After performing heterogeneity testing, the rate of heterogeneity in the current study was 44.43%, which tended to moderate heterogeneity. Therefore, the random effect model was used. Subgroup analysis based on diagnostic methods and bipolar and unipolar disorders was used to detect the source of heterogeneity. Another possible source of heterogeneity was sample size and different types of mood disorders, which was not assessed in the current study. Subgroup analysis was conducted in patients with bipolar or unipolar depressive disorders, which was not significant in unipolar disorders and was significant in bipolar disorders. However, this result may be affected by the limited number of studies and small sample size.
Studies showed that humans are exposed to BDV (24). Evidence of the association between BDV infection and psychiatric disorders was observed in the study by Azami et al. By analyzing 35 studies, significant association was confirmed between BDV infection and schizophrenia, and the estimated OR was 2.72 (95%CI: 1.75 - 4.20) (25). In another meta-analysis conducted in 2017 on 9 studies, a significant association was observed between BDV infection and chronic fatigue syndrome, and the estimated OR was 10.41 (95%CI: 4.24 - 25.55, P < 0.0001) (26). In addition, significant association was noted between BDV infection and depression in a meta-analysis by Wang et al. (27). However, no evidence of BDV infection was observed in patients with mental illnesses in a meta-analysis by Durrwald, suggesting the possible role of sample contamination in the result of previous studies (28).
One of limitations of the current study was lack of access to full texts of several articles. In addition, studies included in the analysis did not use the same methods, samples, and diagnostic indices, which may affect the results. Moreover, studies conducted on this issue were restricted to specific geographical regions. However, it was tried to use all studies conducted on the association between BDV and mood disorders and also the role of different methods in the final results using subgroup analysis. These variables may influence the result of the current and other studies, and their sensitivity and specificity were not similar to those of the study by Billich. According to the findings of the current study, the results of IFA and peptide array were different, which indicated lower sensitivity, but higher specificity of peptide array (29). However, according to the study by Fukuda using different methods such as ECLIA, RT-PCR, and WB in different samples of plasma and PBMC (peripheral blood mononuclear cell), the differences were not statistically significant (30). According to the study by Na, BDV was not detected in any of the subjects in the case or control groups using IFA and RT-PCR methods (15).
6. Conclusions
The current study confirmed the association between BDV infection and mood disorders and suggested the role of viral infections in the etiology of neuropsychiatric disorders. However, more studies in different regions of the world should be conducted to find the exact association and other etiologic agents should be determined. However, reliable diagnostic methods should be introduced. Understanding the underlying factors may help in prevention, diagnosis, and management of patients with psychiatric disorders and may help to reduce the rate of such disorders and their socio-economical burdens.
| Authors Name | Year | Place | Case | Control | Assay | Technique/Sample | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| BDV Positive | BDV Negative | Male/Female | Mean Age (SD) | BDV Positive | BDV Negative | Male/Female | Mean Age (SD) | |||||
| Yamaguchi et al. (31) | 1999 | Japan | 9 | 242 | 98/153 | 55.6 (14) | 10 | 907 | 632/285 | 35.1 (13.1) | Anti p24 and p40 Ab | ECLIA/Serum |
| Amesterdam et al. (32) | 1985 | USA | 12 | 253 | 0 | 105 | Anti BDV Ab | IFA/Serum | ||||
| Fu et al. (33) | 1993 | USA | 9 | 129 | 41/97 | 40 (13) | 1 | 116 | 77/40 | 34 (10) | Anti 38/40 and 24kDa protein Ab | WB/Serum |
| Terayama et al. (34) | 2003 | Japan | 9 (8MDD,1 BD) | 24 (12MDD,12 BD) | 17/16 | 1 | 24 | Anti p10 Ab | WB/Serum | |||
| Tsuji et al. (16) | 2000 | Japan | 0 | 5 | 1/4 | 58.6 (17.6) | 1 | 172 | 110/63 | p24, p40RNA | RT-PCR/PBMCS | |
| Tsuji et al. (16) | 2000 | Japan | 0 | 19 | 6/13 | 50.7 (14.2) | 0 | 210 | 129/81 | Anti p24, p40 or gp18 Ab | WB/Plasma | |
| Matsunaga et al. (35) | 2008 | Japan | 25 | 104 | 38/91 | 49.5(16.4) | 67 | 209 | Anti p40, p24Ab | RLA/Serum | ||
| Billich et al. (29) | 2002 | Germany | 8 | 11 | 4/15 | 53 | 14 | 50 | IFA/Serum | |||
| Billich (29) | 2002 | Germany | 4 | 15 | 4/15 | 53 | 7 | 57 | N,P proteins | Peptide array/Serum | ||
| de la Torre et al. (36) | 1996 | Germany | 3 | 0 | 0 | 5 | p24RNA | RT-PCR/PBMCS | ||||
| Fukuda et al. (30) | 2001 | Japan | 1 | 44 | 23/22 | 47 | 0 | 45 | 22/23 | 48 | Anti p24, p40 Ab | WB/ Plasma |
| Fukuda et al. (30) | 2001 | Japan | 1 | 44 | 23/22 | 47 | 0 | 45 | 22/23 | 48 | P24 RNA | NestedRT-PCR/PBMCS |
| Fukuda et al. (30) | 2001 | Japan | 1 | 44 | 23/22 | 47 | 1 | 44 | 22/23 | 48 | Anti p24, p40 Ab | ECLIA/Plasma |
| Fukuda et al. (30) | 2001 | Japan | 4 | 41 | 23/22 | 47 | 1 | 44 | 22/23 | 48 | p24, p40 proteins | Proliferation assay/Plasma |
| Na et al. (15) | 2009 | Korea | 0 | 138 (98MDD,40BD) | 0 | 60 | Anti BDV Ab | IFA/Serum | ||||
| Na et al. (15) | 2009 | Korea | 0 | 138 (98MDD,40BD) | 0 | 60 | p24, p40RNA | RT-PCR/PBMCS | ||||
| Sauder et al. (37) | 1996 | Germany | 6 | 46 | 3 | 200 | antiP24, p40,p16Ab | WB/Serum | ||||
| Planz et al. (38) | 1999 | Germany | 2 | 0 | 0 | 3 | P40RNA | RT-PCR/Granulocytes | ||||
| Salvator et al. (39) | 1997 | USA | 2 (BD) | 9 (6MDD, 3BD) | 0 | 10 | P24RNA | RT-PCR/Brain | ||||
| Iwata et al. (40) | 1998 | Kubo | 2 | 47 | 25/24 | 47.5 (12.6) | 2 | 82 | 49/35 | 45.2 (10.2) | P24RNA | RT-PCR/PBMCS |
| Czygon et al. (41) | 1999 | Germany | 0 | 11 | 4/7 | 52 (15) | 0 | 52 | 32/28 | 54 (24) | p24, p40RNA | RT-PCR/Brain |
| Nunes et al. (42) | 2008 | Brazil | 9 | 15 | 8/16 | 38.4 (10) | 4 | 23 | 14/13 | 38.8 (10.3) | P24RNA | RT-PCR/PBMCS |
| Kubo (43) | 1997 | Japan | 1 | 122 | 42/81 | 52.95 (13.24) | 0 | 70 | 37/33 | 45.43 (11.04) | Anti BDV Ab | IFA/plasma |
| Matsunaga et al. (44) | 2005 | Japan | 6 | 74 | 22/58 | 46 (16.9) | 1 | 40 | 19/22 | 45.5 (13.9) | Anti BDV-P and N Ab | RLA/Serum |
| Tehrani (17) | 2014 | Iran | 5 (BD) | 76 (12MDD, 59BD) | 37/44 | 8 | 192 | 111/89 | Anti BDV Ab | EIA/Plasma | ||
| Tehrani et al. (17) | 2014 | Iran | 37 (6MDD, 29BD) | 44 (6MDD, 36BD) | 37/44 | 59 | 141 | 111/89 | CIC | EIA/Plasma | ||
| Tehrani et al. (17) | 2014 | Iran | 0 | 81 | 37/44 | 2 | 198 | 111/89 | N,P proteins | EIA/Plasma | ||
| Horing et al. (18) | 2012 | USA | 2 (BD) | 144 (80MDD,64BD) | 1 | 145 | Anti BDV Ab | IFA/ Serum | ||||
| Horing et al. (18) | 2012 | USA | 0 | 146 | 0 | 146 | Anti BDV-P and N Ab | ELISA/Serum | ||||
| Horing et al. (18) | 2012 | USA | 0 | 146 | 0 | 146 | P23,P40 RNA | RT-PCR/WBC | ||||
| Shankar et al. (45) | 1992 | USA | 0 | 5 | 0 | 20 | BDV RNA | RT-PCR/Brain | ||||
| Zhao et al. (46) | 2007 | China | 3 | 57 | 0 | 120 | P24 RNA | RT-PCR/PBMCS | ||||
| Wang et al. (47) | 2006 | China | 6 | 47 | 0 | 32 | P24 RNA | RT-PCR/PBMCS | ||||
| Flower et al. (48) | 2008 | Australia | 5 | 99 | 5 | 214 | Anti p24 and p40Ab | ELISA/Serum | ||||
| Flower et al. (48) | 2008 | Australia | 1 | 103 | 2 | 217 | P24 and p40 pAg | ELISA/Serum | ||||
| Bode et al. (49) | 1992 | Germany | 12 (5MDD,7BD) | 538 (358MDD,180BD) | 45 (13) | 11 | 472 | Anti38/80KDAb | IFA/Serum | |||
| Bode et al. (13) | 1995 | Germany | 2 (MDD) | 1 (MDD) | ` | 0 | 10 | P24, P40RNA | Nested RT-PCR/PBMCS | |||
| Liu et al. (50) | 2015 | China | 97 (MDD) | 437 (MDD) | 187/347 | 35.99 (12.33) | 176 | 819 | 342/653 | CIC | EIA/Plasma | |
| Liu et al. (50) | 2015 | China | 5 (MDD) | 95 (MDD) | 9 | 171 | Anti BDV ab | EIA/Plasma | ||||
| Liu et al. (50) | 2015 | China | 27 (MDD) | 507 (MDD) | 187/347 | 35.99 (12.33) | 58 | 937 | 342/653 | BDVp24RNA | RT-PCR/PBMCs | |
Abbreviations: antibody (Ab); antigen (Ag); borna disease virus (BDV); borna disease virus nucleoprotein (BDV-N); borna disease virus phosphoprotein (BDV-P); circulating immunocomplexes (CIC); electrochemiluminescence immunoassay (ECLIA); indirect immunofluorescence antibody (IFA); nested polymerase chain reaction (Nested PCR); peripheral blood mononuclear cell (PBMC); radioligand assay (RLA); reverse transcriptase polymerase chain reaction (RT-PCR); standard deviation (SD); western blot (WB); white blood cell (WBC).