1. Introduction
Cerebral palsy (CP) is a disabling clinical condition that has led to childhood functional and developmental disabilities (1, 2). Despite considerable advances in medical care of affected infants, the overall incidence of CP has remained unchanged within the recent decades (3, 4). This clinical event could limit children’s daily functional abilities and disturb their health-related quality of life, and lead to death in case of deterioration of clinical conditions as well as appearing concurrent clinical complications, such as neurological, cardiovascular, or respiratory complications (5, 6). Furthermore, this disability could be accompanied by mental retardation because of reduced oxygen and also further decrease of supply to neural pathways and neuronal structure (7). The fundamental treatment of CP is the individualized multidisciplinary approach (8). A large part of physical disabilities can be improved by rehabilitation therapy. Some medications are also indicated to relieve seizures and movement difficulties (9). In some cases, neurosurgical and orthopedic surgical interventions could be helpful (10). However, none of the pointed interventions could fully relieve symptoms of CP or donate natural living health in affected children.
The major effect of a lack of oxygen supply is delay in brain development in children with CP leading to cellular vulnerability due to hypoxic-ischemic insult. Consequently, recent efforts have focused on cellular recovery in children with CP. In this regard, the use of stem cells, as the mother of unspecialized cells, transformable to specialized cells, is currently the subject of much attention (11). In fact, through differentiation of unspecialized cells to brain cells, muscle cells, or heart cells, the process of reducing disability in CP children could be accelerated (12). Nowadays, the current knowledge on how to use stem cells therapeutically has improved. In fact, it is now expected to develop ways to improve damaged cells by transplanting stem cells to cure CP-related injuries (13, 14). The present review aimed at assessing clinical trials related to beneficial effects of stem cells in patients with CP.
2. Methods
2.1. Research Purpose
The main aim of this meta-analysis was to find the efficacy of stem cell therapy in improvement of symptoms in patients with CP assessed by the gross motor function classification system (GMFCS) scale.
2.2. Literature Search Methods
For these aims, a computerized search of articles published in English was conducted by the 2 authors, independently. The following data bases were used for articles published until February 2016: PubMed, Ovid, Web of Science, Scopus, Cochrane library, and CINAHL. The searched key words were stem cell/cerebral palsy/cell therapy/ GMSCS scale, alone or in combination. Then titles and abstracts were screened and all irrelevant and duplicated articles were excluded. Only clinical trials were evaluated in details. Final decision on inclusion was made by both researchers, according to the inclusion criteria. These reviewers extracted data separately regarding study details (study design, publication year and patient number), patient characteristics, details of diagnosis of CP and its risk factors.
Papers that focused on the possible effects of stem cell therapy on motor function improvement in patients affected by CP were considered as the inclusion criteria. Exclusion criteria were as follows: review or observational articles and studies with incomplete data or only abstract availability, papers written in any language other than English, and unpublished materials.
Among 77 studies initially reviewed based on the included keywords, only 6 met the study criteria and were finally considered eligible for the review (Figure 1).
2.3. Statistical Analysis
Pooled response rate of stem cell therapy to treat CP was estimated by assessing the percentage of improvement in GMFCS score using a random-effects model. This statistical technique weights individual studies by sample size and variance (both within and between study variance) and yields a pooled point estimate and a 95% CI. This technique was considered an appropriate pooling technique due to the relative heterogeneity of the source population in each study. The research evaluated the presence of heterogeneity across trials by using the I2 statistic. All of the statistical analyses were done in using Stata, version 13.1 (Stata Corp, College Station, TX).
3. Results
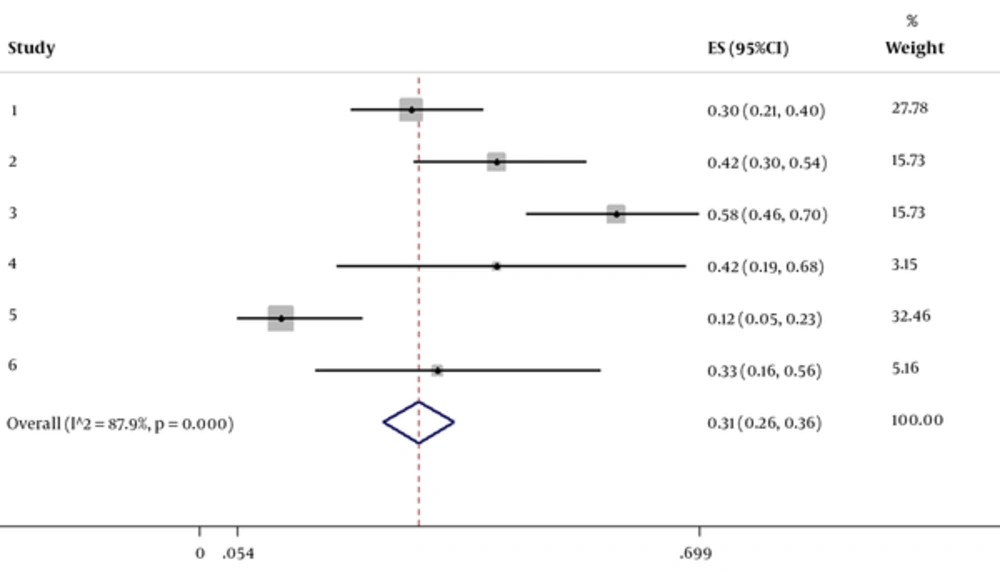
The electronic databases-based reviewing resulted in a total of 77 manuscripts addressing improvement in GMFCS by considering stem cell therapy. There were only six trials fulfilling the inclusion criteria that assessed beneficial effects of stem cell therapy on preserving symptoms in CP individuals. Flow chart of the study selection is shown in Figure 1. The study participants were followed-up for 1 or 6 months. Table 1 presents the main characteristic of 6 eligible studies. One study was performed by Chen et al. (15) in China in 2013, on 60 patients with CP, who were treated with autologous mesenchymal stem cell (MSC) therapy and followed for 12 months. Based on the result of the study, GMFCS score increased up to 42.6% within 3 months and 58.6% within 6 months after completing the treatment protocol. No adverse event was reported by the treatment protocol. The study of Wang et al. (16) in 2013 in china, on 52 patients with CP, who underwent MSC, showed an 11.86-% improvement in Motor score. In Mancias-Guerra et al.’s study (17) in 2013, 18 Mexican patients underwent intrathecal injection of stem cells. In phase 1 of this clinical trial, assessing motor function of the patients after injection versus before treatment revealed 35.35% mean improvement in GMFCS score. In a studies by Zali et al. (18) in 2014, twelve Iranian children with CP underwent intra-thecal injection of CD133-positive enriched bone marrow progenitor cells. After 6 months of observation and compared to the baseline GMFCS of the patients, results revealed a mean percentage of improvement of about 44.4%.
| Author | Country (Year) | Number of Patients | Cell Source | Delivery | Follow-Up, Mo | Improvement in GMFCS Score (95%CI) | Weight, % |
|---|---|---|---|---|---|---|---|
| Chen et al. (15) | China (2013) | 60 | Neural stem cell-like (NSC-like) cells | Intrathecal | 12 | 41.7 (30.06 - 54.27) | 15.73 |
| Chen et al. (15) | China (2013) | 60 | Neural stem cell-like cells | Intrathecal | 12 | 58.3 (45.73 - 69.94) | 15.73 |
| Wang et al. (16) | China (2013) | 52 | Bone marrow mesenchymal stromal cells | Intrathecal and Intraparenchymal | 6 | 11.5 (5.40 - 22.97) | 32.46 |
| Mancias-Guerra et al. (17) | Mexico (2013) | 18 | autologous bonemarrow nucleated cells | Intrathecal and Intravenous | 6 | 33.3 (16.28 - 56.25) | 5.16 |
| Zali et al. (18) | Iran (2014) | 12 | Bone marrow-derived CD133 | Intrathecal | 6 | 41.7 (19.33 - 68.05) | 3.15 |
| Shroff et al. (19) | India (2014) | 91 | Human embryonic stem cells | Intramuscular and Intravenous | 6 | 29.7 (21.26 - 39.72) | 27.78 |
aHeterogeneity chi^2 = 41.22 (df. = 5), P < 0.0001.
bI2 (variation in ES attributable to heterogeneity) = 87.9%
Another study published by Shroff et al. (19) in India during year 2014 on 91 patients in the age range of 2 to 18 years, who underwent a four-phase human embryonic stem cell therapy and followed for 1 to 2 months, assessed the final improvement in motor functional status. In their study, during phase 1 (including 91 patients), 0.25 mL of Human Embryonic Stem Cells (hESCs) were administered intramuscularly once daily plus 1 mL of hESC intravenously twice every 7 days for a total of 8 weeks. In phases 2 and 3 (prolonged for 4 weeks), drugs were administered with the same dosage regiment yet with a gap period of 3 to 6 months and including 66 and 38 patients, respectively. Finally, phase 4 was done after a gap period of 6 to 12 months. The dosage regimen of this phase was similar to that of phase 2, yet the intravenous dose of hESC was increased by 1 mL. After completing the study protocol, 30.2% of participants achieved good motor function indicated using a GMFCS score of 1 within the study period. The treatment protocol had no adverse complications.
The pooled percentage change in GMFCS score was statistically significant in favor of stem cell therapy. The resulting pooled estimate indicated a 30.7% (95% CI: 25.80 % to 35.69 %) increase in GMFCS score 1 to 6 months after stem cell therapy. In this regard, the test for heterogeneity was statistically significant (I2 = 87.9 %, χ2 = 41.22, P = 0.000) (Figure 1).
4. Discussion
Reviewing valid literature shows limited studies on efficacy of stem cell therapy on improving CP. Based on the literature search, only 6 clinical trials were performed to assess changes in motor functional status in CP patients, while most published reports were limited to case reports indicating significant improvement in CP manifestations. This study tried to find trials on beneficial effects of stem cell therapy and finally showed improvement of GMFCS score up to 41.1% within 1 to 6 months after completing the treatment protocols. On the other hand, stem cell therapy with the different protocols, including autologous MSC therapy or human embryonic stem cell therapy, could result in an increase of motor function in patients with CP. However, considering these assessed trials, indicated a significant heterogeneity between the studies (15-19). In fact, because of the difference in treatment protocols as well as different gap periods between treatment phases, the change in motor function was found to be widely varied between the studies. Thus, to achieve a valid pooled motor function change following stem cell therapy in CP patients, further trials with the same protocols should be performed. However, the present clinical trials and case reports could demonstrate beneficial effects of stem cell therapy on motor function in patients with CP (15-20).
Some preclinical studies have examined various types of stem cells, such as MSCs, Schwann cells, and neural precursor cells and finally showed high functional efficacy of these stem cells in neurological regeneration (21, 22). In this regard, it seems that the use of autologous stem cell transplantation could improve most clinical aspects of disabilities in patients with CP. It has been revealed that transplanting autologous bone-marrow-derived mononuclear cells could improve motor, sensory, cognitive, and speech abilities as well as control bowel and bladder motilities (23).
However, the exact mechanism underlying effects of stem cells on neural recovery remains unclear. Some physiological processes have been suggested to be related to this recovery process. First, MSCs could significantly integrate with neural host cells, establish synaptic connections, and recover nerve cell functions (24-26). In addition, MSCs could produce some neurotrophic factors facilitating recovery of disturbed neuronal tissues in affected regions (27-30). In fact, different types of stem cells, such as MSCs, could differentiate into neuronal and glial lineages.
It is better to design a study to collect data on one kind of stem cell for use in clinical trials as different kinds of stem cells behave differently.
4.1. Conclusion
Limited available evidence show therapeutic effects of stem cells on recovery of motor functions in patients with CP. According to the current meta-analysis, the results of the clinical trials led to heterogeneous findings may be due to using different types of stem cells, different therapeutic protocols as well as different gap periods between the phases of treatment. In total, the published experiments, clinical trials, and case reports suggest the potential effect of stem cells transplantation on improving motor function in patients with CP.