1. Context
Cocaine affects the autonomic nervous system (ANS). Cardiovascular effects of cocaine have been extensively studied. The major pharmacological property of cocaine that affects the cardiovascular system is its impact on catecholamines (i.e. dopamine and norepinephrine). It blocks the reuptake of catecholamines at the presynaptic level in the central and peripheral nervous systems and increases the release of such substances from both central and peripheral stores, resulting in increased levels of norepinephrine in the vascular smooth muscle, stimulating postsynaptic alpha-receptors, with a subsequent increase in calcium flux and a vasoconstrictor response (1-3). Furthermore, cocaine has a local anesthetic effect on the heart, by blocking the fast sodium channels in the myocardium, resulting in a depression of depolarization and a slowing of conduction velocity (1).
While the effect of cocaine on the cardiovascular system (4) has been extensively studied using measures of blood pressure (BP, e.g. (5)), heart rate (HR, e.g.(1)) or likewise parameters of cardiovascular activity under different conditions, studies on the effect of cocaine on heart rate variability (HRV) are rare. The heart rate variability (HRV) represents the continuous interplay between the sympathetic and parasympathetic branches of the ANS in regulating the HR. In this comprehensive review, we attempt to summarize current findings on the influence of cocaine on time and frequency domain measures of HRV.
2. Evidence Acquisition
2.1. Search Strategy
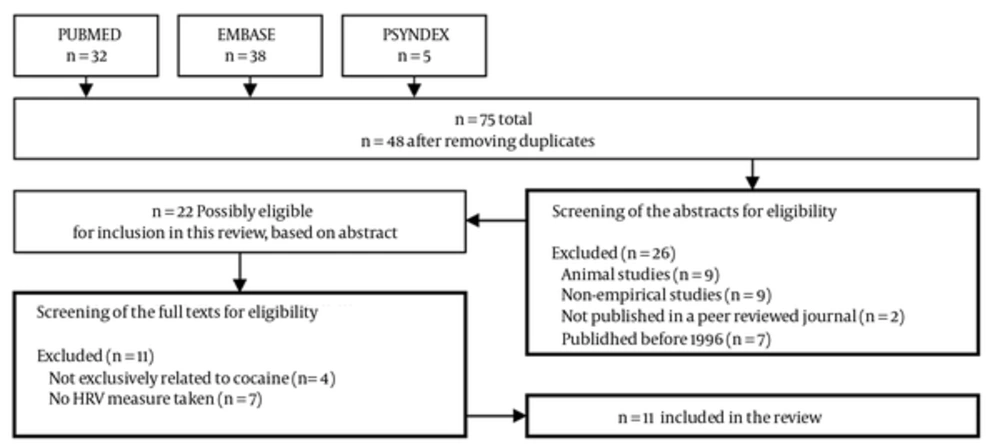
This review uses a systematic approach, according to the “Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA)” statement (6), to extract research studies on the association between HRV and acute cocaine intake. The following computerized databases were searched from 1st of January 1996 up to the 31st December 2012: PUBMED (MEDLINE), PSYNDEX and EMBASE. The search was restricted to publications published within this time frame, since the first guideline on standards of measurements, physiological interpretation and clinical use of HRV was published in 1996 (7). Articles were considered for inclusion if they had a focus on cocaine (search term keyword: “cocaine”) and (AND) measured HRV (search term keyword: “heart rate variability” OR “HRV”). Details were recorded regarding the number of studies found in the databases using the search terms as depicted within the flow-chart (Figure 1).
The abstracts of the manuscripts were then screened for eligibility independently by two authors (JK and MNJ). Differences in initial study identification and selection for review were compared and deviations were discussed until a consensus on the disposition of each under the study could be reached. The screening was based on the following criteria: (a) empirical investigation with HRV measures obtained in human subjects; (b) specifically cocaine related (i.e. reported acute cocaine intake as independent variable or long-term impact of cocaine); (c) published in a peer-reviewed journal; and (d) published in English. Included papers were reviewed in full text for information on (1) study design and subjects, (2) details on cocaine use (i.e. dose, method to determine long-term cocaine intake) (3) method of HRV measurement, and (4) data on HRV time-and (5) frequency-domain measures. The few differences in the evaluation were addressed, reaching a consensus presented in Figure 1. The number of studies meeting the pre-specified inclusion criteria, number of studies excluded, and reasons for exclusion were recorded.
2.2. Data Extraction
Study information on author, country, study population, sample size, gender ratio, age of participants, study design, and main study focus, were extracted from the papers retrieved in full-text. Furthermore, details regarding the method of cocaine intake, the cocaine dose, the method of HRV recording, available data length for analysis and HRV measures obtained from data sets were extracted and summarized within a comprehensive table. Findings and strength of reported effects were derived from the papers retrieved in full-text.
3. Results
The search in the selected databases revealed a total of 48 articles after removing the duplicates (Figure 1). With a total of 38 hits, EMBASE revealed the most findings when compared to PUBMED (n = 32) and PSYNDEX (n = 5). A total of 48 articles were considered for inclusion in the review after removing duplicates. The abstract of all studies was retrieved for further screening of eligibility. Nine animal studies and eight non-investigational articles (e.g. reviews, comments) were excluded. Furthermore, two papers not published in a peer reviewed journal and seven papers published before 1996 were excluded, based on the specified criteria, leaving 22 articles for further consideration, which were retrieved in full-text. Four papers were excluded because they did not specifically relate to cocaine (i.e. alcohol, cocaine and other drugs). Another seven papers were excluded which were not reporting HRV (i.e. only mean HR); leaving a total of 11 studies included in the systematic review.
3.1 Nature of the Included Studies
Three studies reported results from experimental designs addressing the cardiovascular effects of acute cocaine intake either in healthy volunteers (8), non-treatment-seeking cocaine-experienced volunteers (9), or cocaine dependent men (10). The majority of studies investigated HRV in clinical studies, in particular in prenatal cocaine exposed (PCE) infants (11-18). Table 1 provides an overview of the included studies and the main study focus.
| Authors, (y) [ref] | Country | Population | Cocaine Dose/Application | HRV Measures Taken | Main Study Focus | HRV/Cocaine Related Finding |
|---|---|---|---|---|---|---|
| Garde et al. (2011) (15) | USA | Infants, PCE (n = 9); controls (n = 12) | PCE by maternal self-report or neonatal toxicological urinalysis | SDRR, LF (0.03 and 0.1 Hz), MF (0.1 and 0.2 Hz), HF (0.3 and 2 Hz), LF/HF (here: LF+MF/HF) | Effects of prenatal cocaine exposure on the dynamics of heart rate variability in full-term neonates during sleep | No significant differences on HRV (PCE vs. controls) |
| Haigney et al. (2006) (9) | USA | Non-treatment-seeking cocaine-experienced volunteers (n = 29) | 20 mg and 40mg IV infusions of Cocaine on successive days | HRv, QTm, QTv, QTVI | Effect of intravenous cocaine on QT variability | Dose-dependent increase in QT variability, effect disappeared by 45 minutes after the infusion, HRv decreased significantly at 10 minutes post infusion only |
| Irwin et al. (2007) (10) | USA | Cocaine dependent men (n = 19); controls (n=19) | Cocaine (40 mg) infused over 60s by an indwelling catheter | LF, HF, LF/HF | Effect of cocaine versus placebo on monocyte expression of TNF-α and IL-6, differences between cocaine-dependent men versus control subjects; potential mechanism for decreases of resting and stimulated monocyte expression of TNF-α and IL-6 in cocaine dependence by assessing variations in SNS and PNS | LF/HF and HF in cocaine-dependent volunteers |
| John et al. (2007) (14) | USA | Near-and full-term neonates PCE (n = 21); controls (n = 23) | PCE determined by maternal self-report or neonatal urine toxicology | SDRR, SDDRR, CorSDRR, HF, LF, total power, LF/total, HF/total, LF/HF | Effects of prenatal cocaine exposure on HR and HRV in the presence of orthostatic stress among near-and full-term neonates | SDDRR in PCE in the pre-tilt and intratilt segments compared with controls, different SDDRR peak |
| Mehta et al. (2002) (12) | USA | 2 to 6month-old infants; PCE (n = 71); other drugs (n = 89); no drugs (n = 77) | Exposed to cocaine in-utero | Mean N-N interval, SNN-50, SDNN, SDANN, SDNNi, RMSSD, HF, LF, VLF, ULF, total power | HRV alternations after intrauterine cocaine exposure in older infants (transient or persist); teratogenic effect of cocaine | at birth: SDNN, HF, SNN-50, RMSSD in PCE compared to controls; 2 to 6 months of age: SNN-50, RMSSD, total power in PCE compared to controls |
| Mehta et al. (2002) (13) | USA | 24 to 72hour-old infants, PCE (n = 97); other drugs (n = 111); no drugs (n = 102) | Exposed to cocaine in-utero based on either maternal self-report or toxicology studies | HRVi | Feasibility of using HRVi for clinical applications by assessing its reproducibility and evaluating it in a cohort of infants with in utero cocaine exposure | HRVi in PCE compared to other or no drugs, differences according to degree of cocaine exposure |
| Mehta et al. (2001) (11) | USA | 217 infants; PCE (n = 68); other drugs (n = 77); no drugs (n = 72) | Intrauterine cocaine exposure determined by maternal urine and infant urine and meconium testing for cocaine | RMSSD, SDNN, HF, LF, total power | Autonomic control of the heart in cocaine-exposed infants | SDNN in PCE compared to other or no drugs, HF, LF, total power compared to other drugs, differences according to degree of cocaine exposure |
| Regalado et al. (1996) (16) | USA | Infants 2 weeks of age; PCE (n = 17); controls (n = 14) | PCE determined by maternal self-report or neonatal urine toxicology | Interquartile range of the R-R intervals | Effect of sleep state on cardiorespiratory control in cocaine-exposed infants | Interquartile range of the R-R intervals in QS and REM in PCE compared to controls |
| Regalado et al. (1998) (17) | USA | Full-term infants, PCE (n =11), controls (n = 11) | PCE by maternal self-report or neonatal toxicological urinalysis | HF (0.3 to 2 Hz), MF (0.1 to 0.2 Hz), LF (0.03 to 0.1 Hz), total power (0 to 8 Hz) | Determine what types of heart rate variation account for the increased heart rate variability in neonates with chronic cocaine exposure in utero | Total power in QS and AS in PCE compared to controls; MF, LF in QS in PCE compared to controls; LF in AS in PCE compared to controls |
| Regalado et al. (2001) (18) | USA | Full-term neonates, 2 weeks of age, PCE (n = 15), controls (n = 13) | PCE by maternal self-report or neonatal toxicological urinalysis | HF (0.3 to 2 Hz), MF (0.1 to 0.2 Hz), LF (0.03 to 0.1 Hz), LF/HF (here: LF+MF/HF), total power | Autonomic control of heart rate and respiration during the neonatal period in human infants with prenatal exposure to cocaine | MF, LF, total power in QS and AS in PCE compared to controls; HF in QS in PCE compared to controls |
| Vongpatanasin et al.(2004) (8) | USA | Healthy volunteer subjects (n = 24, 12 menand 12 women; 24 to 44 years of age) | Intranasal administration of cocaine (2 mg/kg) | SDNN, RMSSD, pNN50, HF | Effects of Cocaine on Heart Rate Variability in Healthy Subjects | HF, SDNN, RMSSD, pNN50 afterCocaine administration |
aAbbrevitions: AS, active sleep; BP, blood pressure; BRS,baroreflex sensitivity; DM CocE, double mutant Cocaine esterase; ECG, electrocardiography; HR, heart rate; HRV, heart rate variability; HRVi, HRV triangular index; HRv, mean heart rate variance; IV, intravenously; PCE, Prenatal Cocaine exposure; PNS, parasympathetic nervous system; QT interval, a time between the start of the Q wave and the end of the T wave; QTm, QT interval mean; QTv, QT interval variance; QTVI, normalized QT variability index; QS, quiet sleep; SNS, sympathetic nervous system
3.2. Measures
In addition to the basic measures of the HR, such as beats per minute (BPM), variations in the HR can be evaluated by numerous methods and measures derived. These measures of HRV can be divided into two classes: the time-domain and frequency-domain measures of HRV. The most commonly used measures of HRV are summarized in Table 2.
| Name | Description | Unit | ANS Branch |
|---|---|---|---|
| HR | Simple heart rate in beats per minute | BPM | |
| IBI | Raw time of R-wave to R-wave intervals in milliseconds time | ms | Mixed |
| Time Domain Measures: Based on the Interbeat Intervals Directly or on Differences Between Successive Interbeat Intervals. In Addition, There Are Both Short-Term and Long-Term Indices. | |||
| NN | Normal-to-normal intervals | ms | Mixed |
| SDNN | Standard deviation of all N-N intervals | ms | Mixed |
| SDANN | Standard deviation of the average of N-N intervals for each 5-minute period over 24 hours | ms | Mixed |
| pNN50 | Percentage of adjacent cycles that are greater than 50 ms apart | % | primalyvagally mediated |
| RMSSD | Root Mean Square of Successive Differences, in milliseconds. This index acts like a high pass filter, thus removing long-term trends and slower-frequency variability from the signal. Because of the frequency characteristics of the autonomic influences on the heart such that vagal influences cover the full frequency range and sym-pathetic influences are primarily restricted to the lower frequencies, RMSSD reflects primarily vagal influences. | ms | Primarily vagally mediated |
| Frequency Domain Measures: Frequency Domain Analysis Yields Information About the Amount of Variance or Power in the Heart Rate or Heart Period Time Series Explained by Periodic Oscillations at Various Frequencies. Power Spectral Analysis of the Time Series Provides Basic Information on the Amount of Variance or Power as a Function of Frequency (Task Force, 1996). | |||
| HF | High Frequency (0.15-0.4 Hz) | ms2 | primalyvagally mediated |
| LF | Low Frequency (0.04-0.15 Hz) | ms2 | baroreflex activity |
| VLF | Very low frequency (0.003–0.04 Hz) | ms2 | Mixed |
| ULF | Ultra-low frequency (< 0.003Hz) | ms2 | Mixed |
| TP | Total power represent the variance of the measured signal around its mean value exactly equal to the time domain variance of the HR time series | ms2 | Mixed |
| normalization | The so-called normalized scores represent the relative value of each power component proportional to the total power. In addition, the VLF or DC component is often subtracted from the total power in calculating the normalized values. The DC component is defined as the spectral components with a frequency less than 0.03 Hz. The LF-to-HF ratio (LF/HF) has been proposed to reflect the sympathovagal balance (also see below) | - | Mixed |
| LF/HF | LF-to-HF ratio may provide some insight into the relative relations between autonomic inputs. | - | baroreflex activity |
3.3. Time Domains
Time-domain measures can be derived from direct measurements of the normal-to-normal intervals (NN intervals) or instantaneous HR, or from the differences between NN intervals. Within the included studies, reported time-domain measures include the mean NN interval in milliseconds (ms) (12), the mean standard deviation (SD) of all NN intervals (SDRR or SDNN in ms, (8, 11, 12, 14, 15), the square root of the mean of the sum of the squares of differences between adjacent NN intervals (RMSSD in ms, (8, 11, 12), the mean of the SDNN of all NN intervals (SDNN index or SDNNi in ms(12)), or the number of pairs of adjacent NN intervals differing by more than 50 ms divided by the total number of all NN intervals (pNN50 in %, (8), or SNN-50 (12)). Besides these frequently used, authors reported the corrected SDRR (corSDRR, SDRR corrected for HR as described in (14), and SD of differences between successive R-R intervals (SDDRR [14]), the SD of the average of valid N-N intervals (SDANN, (12)), or the inter-quartile range of the R-R intervals (16).
3.4. Frequency Domains
Parametric and nonparametric methods to analysis the power spectral density (PSD) of HRV, allow the calculation of different spectral components of short and long term recordings of HRV. From short-term recordings, three different main spectral components are distinguished: very low frequency (VLF ≤ 0.04 Hz), low frequency (LF, usually 0.04-0.15 Hz), and high frequency (HF, usually 0.15-0.4 Hz) components. Furthermore, an ultralow frequency component (ULF) can be derived from the spectral analysis in long-term recordings (e.g. 24 hours). Depending on the length of the recording different frequency-domain measures with different frequency bands (see Table 1) are reported within the included studies including the power in HF (8, 10-12, 14, 17, 18); the power in LF (10-12, 14, 15, 17, 18); the power in ULF (12), or total power (11, 12, 14, 17, 18). Besides these, several studies include a mid-frequency-band (MF [15, 17, 18]) or a very-low-frequency band (VLF) besides the ULF (12). Traditionally, the ratio between LF and HF (LF/HF ratio) serves as another measure of HRV and was used frequently by the included studies (10, 14, 15). Furthermore, several studies report other ratios, such as the LF+MF/HF [15, 18] the LF/total or HF/total (14). Besides these frequently used, one study reports the mean heart rate (HRm) and variance (HRv), and QT interval mean (QTm) and variance (QTv), and a normalized QT variability index (QTVI), as described elsewhere (9). Furthermore, a single study reports the geometric triangular index (HRVi), which is the total number of all N-N intervals divided by the height of the histogram of all N-N intervals measured on a discrete scale with bins of 7.8125 ms, and with no adjustment for recording length (13).
3.5. Experimental Studies
Three experimental studies investigated the impact of cocaine administration on HRV. While one study included non-treatment-seeking cocaine-experienced volunteers (9), another included cocaine dependent men (10), and another administered cocaine to healthy volunteer subjects (8). The studies also differed by the dose of cocaine administered (Table 1). The study by Haigney et al. (9) revealed a dose-dependent increase in QT variability, which disappeared by 45 minutes after the infusion. The authors found that the mean heart rate variance (HRv) decreased significantly at 10 minutes post cocaine infusion, indicating a depression in normalized HRV. The authors argue that cocaine reduces vagal activity, thereby reducing variability of the HR, which can potentiate cocaine’s sympathomimetic effects. As these effects were manifest by 10 minutes but were not temporally disconnected from the peal increase in QT variance, the authors suggest that modulation of autonomic tone accounts only for a part of cocaine’s proarrhythmic effects (9).
Irwin et al. (10) found, that compared with control subjects, cocaine-dependent volunteers had higher levels of the ratio of LF/HF power, along with decreases in the HF power. The authors conclude that these findings indicate a shift toward sympathetic dominance and a withdrawal of parasympathetic activity at rest in cocaine-dependent volunteers compared with healthy control subjects. The study by Vongpatanasin et al. (8) administered intranasal cocaine to healthy volunteers to study effects on HRV. The authors found a significant decrease of HF, SDNN, RMSSD, and pNN50 after Cocaine administration. The authors revealed a striking similarity in the time course of the observed increase in HR and decrease in HF power. The authors state, that they found cocaine causes an unequivocal decrease in HF power, implicating an important vagolytic component to this positive chronotropic response (increase in HR). They suggest a major vagolytic action of cocaine on the human sinus node.
3.6. Clinical Studies
The majority of the included studies had a clinical nature, with all of them investigating the effects of prenatal cocaine exposure on the dynamics of HRV. While one study focused on general HRV difference in neonates with PCE versus controls (11), four studies focused on HRV in PCE neonates during sleep (15-18), one study addressed HRV in PCE near and full-term neonates in the presence of orthostatic stress (14), one studied HRV in two to six month-old infants with PCE (12), and one study assessed the feasibility of a particular HRV measure (HRVi) for clinical applications by assessing its reproducibility in a cohort of infants with in-utero cocaine exposure (13).
The study by Mehta et al. (11) found decreased SDNN, HF, LF, and total power in 24 hour recordings of HRV of PCE neonates compared to neonates exposed to other drugs or none. Furthermore, the authors found differences according to the degree of cocaine exposure. Within a later study by the same authors (13), a decreased HRVi in PCE neonates compared to neonates exposed to other or no drugs was found. Again differences according to the degree of cocaine exposure were found. A third study by these authors (12) investigated HRV alterations after intrauterine cocaine exposure in older infants. In near and full term PCE neonates at birth, they found decreased SDNN, HF, SNN-50, and RMSSD compared to controls. Interestingly, they found increased SNN-50, RMSSD, and total power in PCE infants compared to controls at the age of two to six months. They concluded, that these alterations noted at follow up suggest a possible teratogenic effect of cocaine on the development of ANS.
The majority of studies on PCE neonates focused on HRV during sleep. Within their first study, Regalado et al. report an increased inter-quartile range of the R-R intervals during quite sleep (QS) and REM in PCE neonates compared to controls (16). The authors conclude that these findings of greater HRV across both sleep states in the cocaine exposed infants may be indicative of a less finely-tuned homeostatic system allowing with greater deviations from the norm. A later study by the same group (17) reported an increase in total power during QS and active sleep (AS) in PCE compared to controls. Furthermore, MF, and LF were increased during QS, and LF was increased during AS in PCE compared to controls. The authors state, that such increases in mid and low frequency HRV are suggestive of specific effects of PCE on catecholamine sensitivity and/or metabolism. The other study by the authors (18) supports their earlier findings. Within this study again MF, LF, and total power were increased during QS and AS in PCE neonates compared to controls. Furthermore, HF was increased during QS. The authors suggest two possibilities for the effect of cocaine exposure on the autonomic nature of HR: (1) an increase in parasympathetic control of HT in the cocaine-exposed infants; or (2) an increase in both vagal and sympathetic modulation of HT in the PCE infants. In line with their previous studies, the authors tend to favor the second possibility, since sympathovagal balance was unaffected within this study, and therefore it appears that both parasympathetic and sympathetic activities are increased. However, the study by Garde et al. (15) reports no significant differences on HRV in PCE neonates compared to controls. The authors state that they found large intersubject variability not only in the PCE group but also in the control group. Thus, they critically note, it appears that any alterations in HT dynamics in PCE neonates are likely to be too subtle for detection even by nonlinear techniques unless much larger sample sizes are employed and that this fact might contribute to explain why previous studies using spectral analysis of HRV have arrived at differing conclusions.
John et al. (14) addressed the effects of PCE on HR and HRV in the presence of orthostatic stress among near and fullterm neonates. They found decreased SDDRR in PCE neonates in the pre-tilt and intra-tilt segments compared with controls. Furthermore, PCE neonates had a different and SDDRR peak. The authors conclude that the effects of PCE on the development of SNS and PNS could lead to altered cardiovascular function.
4. Conclusions
Cardiovascular effects of cocaine have been extensively studied by different measures of autonomic nervous system (ANS) function, such as blood pressure (BP), heart rate (HR) or likewise parameters of cardiovascular activity under different conditions. The heart rate variability (HRV) represents the continuous interplay between the sympathetic and parasympathetic branches of the ANS in regulating the HR. Within this comprehensive review, we aimed to summarize current findings on the influence of cocaine on time- and frequency domain measures of HRV.
After extensive screening of the literature, 11 studies were included within the systematic review. Three experimental studies investigating acute effects of cocaine intake/administration in adults and eight clinical studies investigating prenatal cocaine exposure (PCE) in infants were eligible for inclusion. Surprisingly, the amount of experimental studies was relatively small and all clinical studies focused on PCE.
Evidence on the effects of acute cocaine administration on HRV from experimental studies is rare. Of the included studies within this systematic review, only three investigated HRV by an experimental design. Of these, only one intentionally aimed to investigate direct effects of cocaine administration on HRV in healthy subjects (8). The others implemented HRV as additional control variable, to investigate the effect of cocaine versus placebo on monocyte expression and potential mechanism for decreases in resting and stimulated monocyte expression of TNF-α and IL-6 in cocaine dependence by assessing variations in SNS and PNS indexed by HRV (10), or to investigate whether cocaine would significantly destabilize cardiac repolarization as measured by QT variability (9). A common finding among the above studies is a decrease in high frequency (HF) of HRV after cocaine administration as reported by two studies (8, 10). However, the amount of experimental studies is simply too small to draw substantial conclusions based on the present evidence.
All clinical studies investigated the impact of PCE on infants right after birth, 24 to 72hours-old, two weeks of age, or at the age of 2 to 6month. While one study (10) report no significant effect of cocaine on HRV in PCE infants compared to controls, the majority of studies reveal significant differences. Of these, several studies report a decrease of HRV in PCE infants compared to controls (11-14), while others report an increase (16-18). The study by John et al. (14) was motivated by the presence of such conflicting results, and addressed orthostatic stress as potential source of bias. They found that PCE infants had decreased HRV in the pre-tilt and intratilt segments compared with controls, but they had similar or increased HRV in the post-tilt segment. The authors state, that moreover, the effects of cocaine exposure on ANS regulation of cardiovascular function were manifested as differences in the times to peak HRV and the duration of elevated HRV (14). The authors conclude, that these increases in the presence of elevated HR (during tilt) likely reflect both sympathetic and vagal modulation in both PCE and controls, while the lower HRV measurements in PCE compared with controls (pre and intratilt), suggest the predominance of sympathetic modulation in the ANS control of cardiovascular function in PCE (14). While these findings might contribute to an integration of the aforementioned conflicting results, more research seems necessary that in particular controls for stimulus response (e.g. orthostatic stress) and condition at recording. Furthermore, more research is needed to clarify to what extent the effects of prenatal cocaine exposure persist into childhood (12, 14).
A general finding of the present review on the one hand is that evidence on the effects of cocaine on HRV is rare. On the other hand, existing studies encourage the use of methods of HRV measurement to study the impact of cocaine on ANS function. Besides the expansion of current fields of research (i.e. acute administration of cocaine in adults, ANS changes in cocaine dependent subjects, PCE in infants), future studies might investigate changes in HRV as potential outcome in the treatment of addictive subjects, investigate the relation of HRV differences and severity of dependency (i.e. dose-effect relation), or address likewise questions of interest.
This systematic review also revealed that in addition to the frequently used measures of HRV (Table 2) a noticeable amount of different and not so well established indices (e.g. HRVi, HRv, CorSDRR) of HRV were used within the included studies. Comparisons between different studies are therefore sometimes difficult or inappropriate. Furthermore, the interpretation of HRV measures is often unclear. For example, the LF and LF power contrary to conventional wisdom reflect the baroreflex activity rather than the sympathetic activity (19-21). We like to encourage research on the impact of cocaine on HRV, and promote a higher level of standardization, according to the measurement and interpretation of results. This said, HRV is a promising index to study changes in the ANS function within this fascinating field of research.
To conclude, evidence on the effects of cocaine on HRV is rare. Existing studies either address the impact of acute cocaine administration in adults or the effect of PCE in infants of different age groups. Current findings encourage the use of heart rate variability to index the autonomic nervous system function within this particular field of research.
Acknowledgements