1. Background
Depression is a common mental disorder. Globally, 1 in 6 individuals (16%) will experience depression at some time in their life (1). Depression is one of the most important causes of disability in the world that has been marked as a major contributor to the overall global burden of disease (2, 3). It has been reported that this disorder is as costly as heart disease or AIDS to the US economy, resulting in over 51 billion dollars in indirect costs (e.g., for absenteeism from work and lack of productivity) and 26 billion dollars for direct medical costs (4). According to the definition proposed by the American psychiatric association, depression is considered a heterogeneous disorder mostly manifested with symptoms at the behavioral, physiological, and psychological levels (5). Often, patients refuse to take appropriate doses of synthetic antidepressants due to the expected side effects including dry mouth, sexual dysfunction, constipation, and difficulty in driving a car (6). Although there are several applied treatments for depression, the response of depressive patients to these treatments is not remarkable (7). Therefore, finding a cheaper treatment option with fewer side effects and higher compliance may improve patients’ compliance. Gastrointestinal (GI) tract is the largest epithelial surface of the human body and can be contemplated as the largest surface area of exposure and interaction with both intrinsic commensal microorganisms and exogenous pathogens (8). Alteration in gut microflora can affect systemic immune responses even in the central nervous system (CNS). For example, studies demonstrated that stress, in addition to provoking inflammation, increases the intestinal permeability, resulting in increased direct access of exogenous pathogens to CNS and subsequent hypothalamic-pituitary-adrenal (HPA) axis hyper-reactivity (8). In animal studies, modifying gut microbiota enhanced GABAergic and serotoninergic pathways and improved depressive-like and anxiety-like behaviors (9). In a study on 40 patients with major depressive disorder, supplementation with L. acidophilus, L. casei, and B. bifidum (6 × 109) improved Beck depression inventory score (10). Although the effect of gut dysbiosis on host health has received special attention in recent years, studies on the relationship between GI track health and CNS function, especially depression, are rare. We designed the present study to assess the effects of synbiotic supplementation in the treatment of moderate depression in a double- blind, placebo-controlled, randomized trial during 6 weeks.
2. Methods
A total of 40 adult (18 to 55 y) outpatients from university hospital psychiatry clinics, who fulfilled the diagnostic and statistical manual of mental disorders fifth edition (DSM-V) (5) for moderate depression, were recruited based on the structured clinical interview. Patients had Hamilton rating scale for depression (HAM-D 17-item) (11) score of 17 to 23 at baseline according to the expert psychologist interview (12). Patients with the following DSM-V diagnosis were excluded: current or past history of schizophrenia and schizotypal personality disorder, bipolar disorder, and cognitive disorder in the past year. Patients received fluoxetine (20 mg/d) for 4 weeks before study entering the study. Since depression could be a life- threatening disorder and the participants were outpatients, strict safeguards were considered. Thus, the participants were excluded from the study whenever they showed a risk of suicide at any time during the study. Those who scored 2 or more on the suicide item of the HAM-D, or who were believed to have significant suicidal tendency from an investigator point of view were also excluded. Moreover, participants were excluded if they showed any clinically significant worsening in condition from baseline. We also excluded pregnant and lactating women. Written informed consent was obtained from all participants and the protocol satisfied the Shahid Beheshti University of Medical Sciences ethics committee requirements (Ethical code = 1394/S/35029).
2.1. Synbiotic Capsule
The synbiotic (Familact H®) used in this study was donated by Zist Takhmir Co. (Tehran, Iran). Each capsule contains 500 mg probiotics, which precisely consist of the following strains and species: Lactobacillus casaei 3 × 108 CFU/g Lactobacillus acidofilus 2 × 108 CFU/g Lactobacillus bulgarigus 2 × 109 CFU/g Lactobacillus rhamnosus 3 × 108 CFU/g Bifidobacterium breve 2 × 108 CFU/g Bifidobacterium longum 1 × 109 CFU/g Streptococus thermophilus 3 × 108 CFU/g and 100 mg fructooligosaccharide as prebiotic. Placebo contained 1000 mg magnesium stearate.
2.2. Study Design
Patients underwent a standard clinical evaluation including a psychiatric assessment, a well-designed diagnostic interview, and a medical history. In this double- blind, multicenter trial, 40 patients in a 1:1 ratio were randomly given 2 capsules of FamiLact or 2 capsules of placebo for 6 weeks after 4 weeks of treatment with fluoxetine. The patients were recommended to consume these capsules just after lunch and dinner every day. The allocation was kept in sealed envelopes till the time of allocation. The randomization and assignment process was accomplished by Zist Takhmir Co. All the patients completed the trial. Patients were assessed by a psychiatrist at baseline (starting fluoxetine therapy), a after 4 weeks of treating with fluoxetine (stating the intervention), and 3 and 6 weeks after the beginning of synbiotic therapy (at seventh and 10th week of the study period, respectively). The main outcome was the score of 17-item HAM-D. Standardized instructions of HAM-D scoring was used to rate the mean reduction of HAM-D score as a measure of response to therapy. Throughout the study, the person who ordered the medications (psychiatrist), the rater (study researchers), and patients were blind to allocation.
2.3. Side Effects
The rater recorded the side effects throughout the study.
2.4. Statistical Analysis
Considering α = 0.05, β = 0.2, the final difference between the 2 groups, a score of at least 5 of the HAM-D total scores that is clinically detectable, power = 80% and S = 5, the sample size was calculated to be at least 15 in each group. Prior to analyzing the data, the normal distribution of each variable was tested using Kolmogorov-Smirnov test. Thus, independent sample t test was applied to compare normally distributed variable (age) between the 2 studied groups. Furthermore, Fisher’s exact test (two sided) was used to compare the demographic characteristics and the frequency of side effects. Time-treatment interaction was assessed using two-way repeated measures analysis of variance (ANOVA), with a two- tailed post hoc Bonferroni mean comparison test. The within subject factor (time) was the four HAM-D total scores (baseline, week 4, 7, and 10). The 2 groups were considered as a between-subjects factor (group). Differences in HAM-D total scores between synbiotic treated and placebo receiving groups during the study period were examined using analysis of covariance (ANCOVA) to account for gender, age, BMI at baseline, as well as baseline HAM-D total scores. The results were considered significant with P < 0.05. All tests were two-tailed. SPSS 19 (SPSS, Chicago, IL) was used for data analysis.
3. Results
The flow diagram of the studied participants is shown in Figure 1. Of 68 screened patients, 40 were finally enrolled in the trial and randomly allocated to the 2 studied groups. All the participants completed the study. Of the study participants, 70% were female in each group. The mean age of the participants in synbiotic treated group was 34.4 years and it was 35.50 years in placebo receiving group. Regarding the basic demographic characteristics including gender, age, and anthropometric indices (BMI), no significant differences were observed between patients randomly allocated into the synbiotic or placebo groups (Table 1).
3.1. Efficacy: Synbiotic vs. Placebo
The behaviors of the 2 protocols were similar across time. The one-way repeated measures analysis of variance (ANOVA) showed a significant effect of both treatments on HAM-D total scores (P < 0.0001 for within group comparisons). In the both studied groups, post hoc comparisons showed a significant change from Weeks 0 to 4 and 7 to 10 on the HAM-D scores. (Mean changes were -19.25 and -17.75 for synbiotic and placebo groups, respectively, P ≤ 0.0001 for within group comparisons in both groups; Table 2).
| Variable | Base line (Starting Fluoxetine Therapy) | 4th week (Starting synbiotic/ placebo intervention) | 7th week | 10th week | Mean (SD) Changes from baseline to 10th week | P Valueb |
|---|---|---|---|---|---|---|
| Hamilton rating scale for depression (HAM-D) | ||||||
| Synbiotic group | 22.90 (22.08 - 23.72)c | 14.50 (13.43 - 15.57)c | 12.20 (10.94 - 13.46)c | 3.65 (2.92 - 4.38)c | -19.25 (1.71) | 0.000d |
| Placebo group | 22.55 (21.80 - 23.30)c | 14.30 (13.29 - 15.31)c | 12.90 (11.42 - 14.38)c | 4.80 (4.16 - 5.44)c | -17.75 (2.05) | 0.000d |
| P value | 0.550e | 0.953f | 0.241f | 0.013d,f | 0.024d,e |
Changes in Hamilton Rating Scale for Depression (HAM-D) of the Study Subjects in Synbiotic and Placebo Groups at the End of the 3rd and 6th Week of the Study Relative to Baseline (N = 20)a
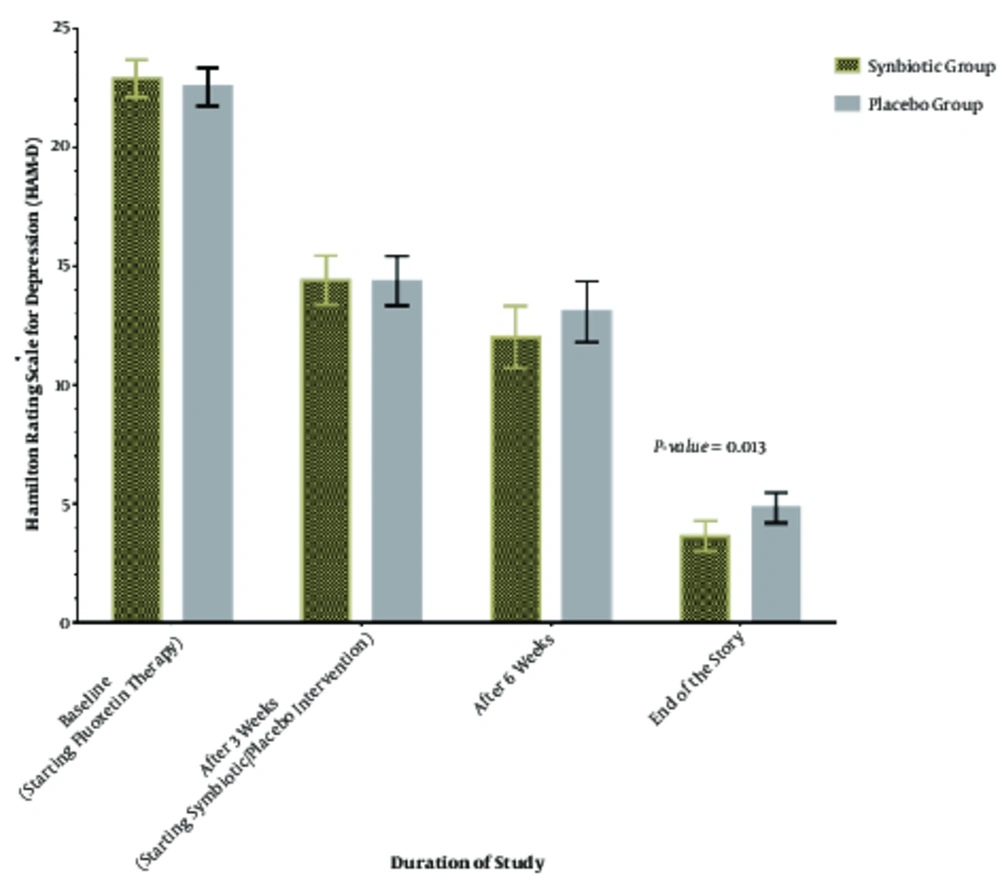
There were not any significant differences between the 2 groups in HAM-D score rated at baseline and after 4 and 7 weeks of the study period (Table 2). However, the difference between the 2 treatments was significant at the endpoint of the intervention. At the end of the 10th week of the study period and after applying ANCOVA for between group comparison adjusted for gender, age, and BMI at baseline, and baseline HAM-D score, it was found that synbiotic group had a significantly lower HAM-D score rating compared with placebo (3.65 vs. 4.80, P = 0.013 for between group comparison; Table 2 and Figure 2).
Furthermore, compared with placebo, synbiotic was significantly more effective in improving HAM-D scores at the end of the study relative to baseline. Thus, following the adjustment of ANCOVA models for gender, age, and BMI at baseline, we found a greater reduction in HAM-D score in synbiotic treated patients (with a mean ± SD of -19.25 ± 1.71) compared to placebo taking group (with a mean ± SD of -17.75 ± 2.05) (P = 0.024) for between group comparison; Table 2 and Figure 2.
Figure 2 displays the time related reduction in HAM-D scores in the 2 studied group at each time point after the intervention.
3.2. Side Effects and Clinical Complication
A total of 4 side effects were observed throughout the trial. As presented in Table 3, in the synbiotic taking group, 20% (4 patients) had bloating and/or nausea, 10% reported diarrhea, and 15% had abdominal cramp. However, the differences were not significant between the synbiotic and placebo groups in the frequency of side effects (Table 3).
4. Discussion
Mental disorders impose an emotional and financial burden on the Western societies. Therefore, the search for new, reachable, and effective therapeutic agents is of great value. There is now compelling evidence for a link between enteric microbiota and brain function (9). The intake of probiotics and prebiotics modulates the inflammation process in the body, which is strongly connected to depression and anxiety, as well as influencing the neuroendocrine response to stress (13). This study showed that patients with moderate depression receiving synbiotic as an adjuvant therapy to fluoxetine, experienced statistically significant HAM-D total score improvement after a 6- week intervention. The clinical relevance of these findings was emphasized by the reduction observed in the HAM-D scores in the synbiotic group. According to a systematic review of randomized controlled trials concerning the effects of probiotic supplementation on the symptoms of anxiety and depression, most of the relevant studies were performed on healthy adults and there was only one study on patients with major depression (14). Our results were in line with a recently published study on the effect of treating healthy adults with conventional yogurt (100 g/day) and 1 placebo capsule (n = 20), or 1 probiotic capsule daily and conventional yogurt (100 g/day) (n = 25), or probiotic yogurt (100 g/day) and 1 placebo capsule (n = 25). They showed that a 6-week intervention with probiotic yogurt and probiotic capsule resulted in significant improvement of depression anxiety and stress scale scores. Probiotic yogurt contained Lactobacillus acidophilus and Bifidobacterium lactis and probiotic capsules were composed of Lactobacillus casaei, Lactobacillus acidofilus, Lactobacillus bulgarigus, Lactobacillus rhamnosus, Bifidobacterium breve, Bifidobacterium, Streptococus thermophilus, and fructooligosaccharide (15). Our results were also in accordance with a study on 132 healthy older adults who consumed a probiotic containing milk or placebo for 3 weeks. In the mentioned study, consuming Lactobacillus casei -containing yoghurt had beneficial effects on the mood of the participants with poor mood at initiation according to the profile of mood states (POMS) (16). As mentioned, our results were in agreement with the results of a recent study on 40 patients with major depressive disorders who were supplemented with L. acidophilus, L. casei, and B.bifidum (6 × 109) for 6 weeks. Probiotic supplementation improved Beck depression inventory score (10). With respect to side effects, the 2 groups did not differ significantly.
Depression is accompanied by symptoms related to impairments in HPA axis including augmented concentrations of corticotrophin releasing factor (CRF) and cortisol in plasma, and cerebrospinal fluid (CSF), respectively. High plasma concentrations of proinflammatory cytokines have also been detected in depressive patients, which are responsible for some of the main demonstrations of the disorder (17). Furthermore, as stated earlier, the role of gut microbiota in the progression of some of the disorders related to the brain and central nervous system (such as anxiety, depression and mood disorders) is not negligible. Dysregulation of GI function, in addition to impairment in the signaling pathways in CNS (such as serotonergic and GABAergic signaling systems) and immune system disturbances, may occur subsequent to dysbiosis of the gut microbiome. These alterations could result in changes in body fat storage, energy homeostasis, intestinal permeability, inflammatory responses, and accelerated stress levels that may lead to disturbances in mood and behavior related disorders, such as depression and anxiety (18, 19).
Thus, there are a number of likely causes for the observed improvement of the depression after synbiotic adjuvant therapy to fluoxetine. First, given the role of HPA axis in regulating moods and behavior, synbiotic therapy can modulate its stress responses and improve depression symptoms. Second, the probiotic contents in synbiotic agents negatively affect the growth of pathogens and their components including lipopolysaccharide (LPS) in the intestine and result in enhancement of the gut microbiota, which might alter the neurotransmitter levels in the CNS (especially GABA and serotonin). Third, suppressing proinflammatory responses by these compounds may enhance the inflammation and disturb immune responses in the disorder (17-19).
In addition, as the previous studies were mainly focused on the effect of consuming probiotics on sad mood in healthy individuals, it is of interest to document the effects of probiotic supplementation in patients with moderate depression. According to the findings of the present study, it seems that synbiotic therapy can be highly promising as an adjuvant therapy to fluoxetine in treating depression. However, our research was limited to the short period of follow-up and small number of participants. Thus, the current results highlight the importance of designing high-quality randomized clinical trials to evaluate the efficacy of probiotic as an adjuvant therapy to other antidepressant treatments.
4.1. Conclusion
The results of the current study indicate the efficacy of synbiotic as an adjuvant therapy to fluoxetine in moderate depression. Additional studies should be conducted in this area to confirm our results.