1. Background
Speakers naturally detect and correct most of the erroneous parts of their own speech, before (inner speech) as well as after they are overtly articulated. This important cognitive ability is referred to as speech monitoring (1). Findings from studies dedicated to the exploration of self-monitoring in pathological speech conditions indicated that individuals with different aphasia syndromes (e g, the Broca, Wernicke, and anomic aphasia) are impaired to some degree in self-detection and self-correction of their verbal errors, during producing either inner or overt speech (2-4). The occurrence of unwanted speech errors and inability to correct them is a major complaint of such patients, especially the ones with better comprehension ability and less severe language impairment (2, 5, 6). Respective monitoring skill was mentioned as an indicator of communication skill and one of the key factors related to language recovery in aphasia (5, 7). Individuals with aphasia who monitor a higher percentage of their own verbal errors benefit more from language interventions (5). Whitney applied a self-monitoring treatment to reduce speech disfluencies in four individuals with mild to moderate aphasia. She trained patients to listen to themselves and monitor their own disfluencies during two picture description conditions: first, audiotaped format, and then online condition. In online condition, patients independently monitored themselves and stopped the occurrence of disfluency. In addition to improvement of communication efficiency, increasing individuals’ self-awareness about the quality of their speech was the main finding of the study (8). A recent study conducted by Schwartz et al. also supported the regulative effect of spontaneous self-monitoring on the speech production system in aphasia (9). Furthermore, the naming therapy methods developed based on errorful learning and self-cue strategies consider the valuable role of verbal self-monitoring in aphasia (10, 11).
In the last decade, transcranial direct current stimulation (tDCS) as a non-invasive neuromodulatory technique suited to enhance or reduce activities of brain areas is adopted in aphasia rehabilitation (12). Despite differences between tDCS studies in post-stroke aphasia, including stimulation parameters (such as session duration, electrode montage) and patients characteristics, the results generally suggest an effectiveness of tDCS over language-related brain areas on language recovery (12, 13). The current study aimed at applying tDCS over a central brain region responsible for speech monitoring in individuals with aphasia.
Recently, functional imaging techniques and electrophysiological evidence (14, 15), in addition to computational modeling of speech monitoring in aphasics (16) support the involvement of a general-purpose error detection system (responsible for all actions) in speech error monitoring. This system is hypothesized to be located in the medial frontal cortex (MFC), particularly the anterior cingulate cortex (ACC).
The role of the MFC in conflict monitoring, as well as performance adjustments and learning processes (17), is explored in clinical investigations concerning patients with deficient error-monitoring ability other than aphasia. Reinhart et al., applied tDCS over the MFC in patients with schizophrenia and observed an alteration of the electrophysiological correlates of error detection and subsequent enhanced learning from mistakes (18). There is no similar protocol for verbal monitoring in aphasia. Considering the assumed important role of self-monitoring in quality of the speech production, it is hypothesized that anodal tDCS over the ACC affects speech self-monitoring skill in aphasia. In the current study, stimulation is combined with the online performance of inner and overt speech monitoring tasks to enhance the targeted efficacy of the intervention.
2. Methods
2.1. Study Design
The current two-factorial (two stimulation types: anodal or sham × 2 monitoring channels: internal or external), randomized, sham-controlled, double-blind, and within-subject experiment will be conducted on individuals with non-fluent aphasia.
In this design, a group of patients meeting the inclusion criteria will complete four different (based on the above-mentioned factors) conditions during four single sessions, separately. Control for possible order effects, the sessions are presented to the patients randomly in one of the ABCD/BCDA/CDAB/DABC sequences, according to the rotation strategy (19). Each active anodal condition will be controlled by its equivalent sham stimulation. Since the current study will be double-blind, the patient and the examiner will be not aware of the type of tDCS polarity. In each session, the concurrent and short-term after-effects of the brain stimulation will be evaluated.
2.2. Participants
Participants will be patients with stroke and non-fluent aphasia recruited from rehabilitation centers in Tehran, Iran. The patients are diagnosed with aphasia based on the neurological examinations and the Persian version of the Mississippi aphasia screening test results (20). After that, the individuals with aphasia will be included in the study if they fulfil the following criteria: a single left hemispheric ischemic stroke (as documented by MRI/CT scans) following left middle cerebral artery (MCA) involvement, age range 18 to 65 years, at least six months post-stroke, fluent in Farsi speaking before stroke, right-handed, and normal or corrected-to-normal vision and hearing. Patients have to be able to understand instructions and produce single words at least, and have a naming disorder, naming accuracy of 5% - 70% in version “A” of the parallel picture-naming tests (PPNTs) (21).
Individuals with a confounding diagnosis of traumatic brain injury, dementia, or mental illness, severe dysarthria, severe apraxia, visual or auditory agnosia, history of seizures, implanted metal objects, and heart problems will be excluded. Smoking status of all participants will be obtained and participants will be instructed to avoid caffeine and alcohol consumption during the tDCS sessions.
2.3. Informed Consent
A written informed consent will be obtained from each participant agreed to take part in the study by the same speech therapist administrating all steps of the current study before participation.
The current study protocol was approved by the ethics committee of Tehran University of Medical Sciences (TUMS), Tehran, Iran.
2.4. Sample Size
Sample size was determined based on a pilot study of five individuals receiving all four sessions of the experiment. The estimated effect size of primary dependent variables across tDCS conditions was calculated. Then, the sample size expected to result in a significant outcome with 80% power at the 0.05 significance level was determined using G*Power 3.0.10 software for the primary statistics, a repeated measures ANOVA. Regarding a reasonable effect size for different primary outcome measures, a sample size of at least 20 participants was expected.
2.5. Materials
Self-monitoring performance of speech production will be tested under two conditions of picture naming: a) overt speech monitoring task, in a normal auditory feedback condition, and b) inner speech monitoring task, in a noise-masked condition.
Since each condition is presented to the participants twice, four versions of the picture naming task were prepared. Each version contains 108 black and white line-drawings assigned to Persian nouns taken from the PPNTs (21). They are presented on a PC screen for 20 seconds, at 500 milliseconds intervals. In all sessions, half of the items of each version are tested during (online), and half of them after tDCS (offline). Stimuli in all versions, as well as the on- and offline parts of each version are matched for psycholinguistic properties (e g, word frequency, length of names, and age of acquisition).
In the inner speech monitoring condition, white noise is generated by a noise generator (Audio Sweepgen software, version 3.7.6.38) and presented to the individuals through headphones at a level of 90 dB. The noise is started at 60 and gradually increased to 90 dB not to let the individual hear his/her own voice (4).
In these tasks, participants are instructed to name each picture after its presentation on the screen. There is no cue/or instruction for self-correction during tasks, but patients will have enough time to monitor their responses.
2.6. Procedures
The study will be implemented at the neurostimulation lab, department of speech therapy, school of rehabilitation, TUMS, Tehran, Iran. The test will be conducted for each patient separately.
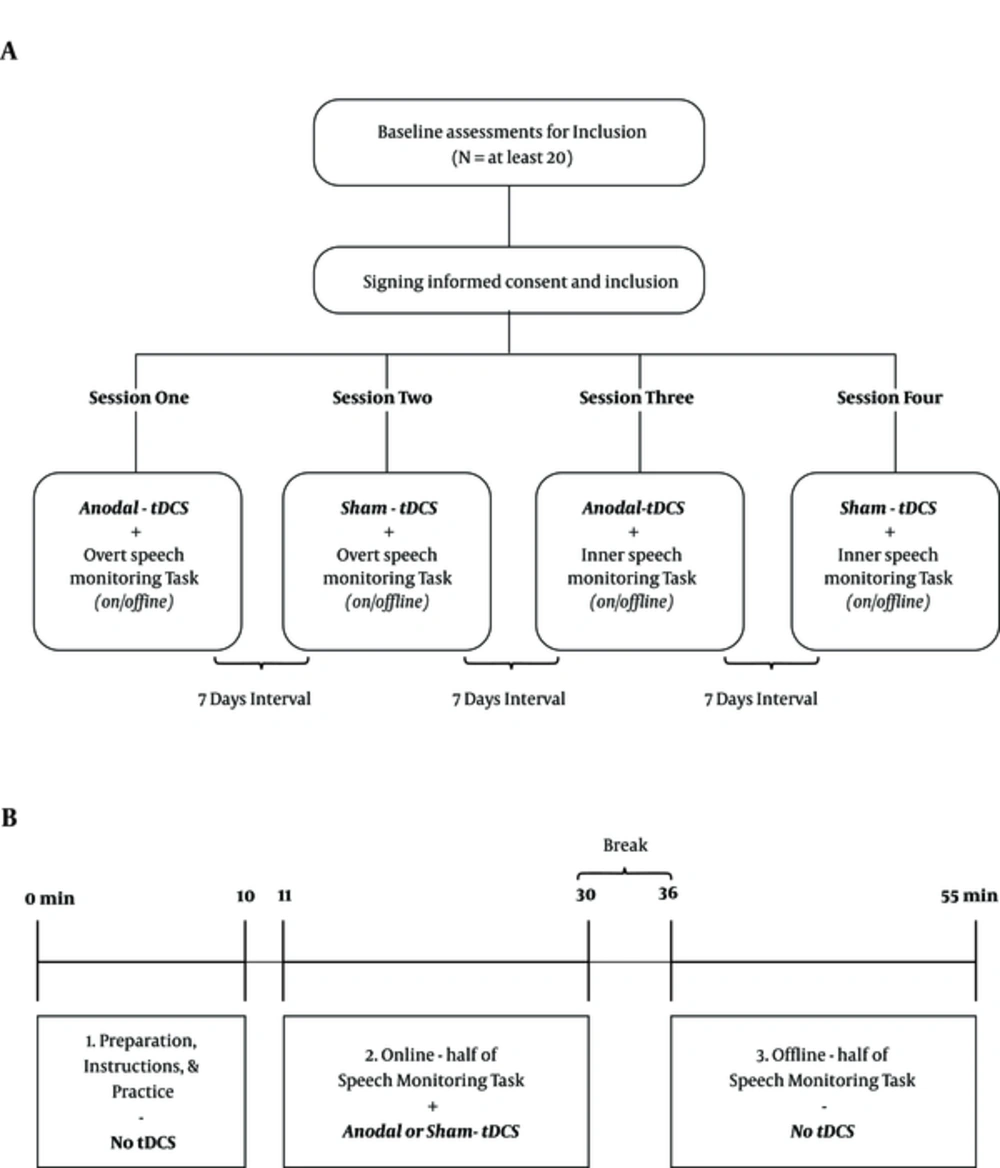
In general, four different sessions will be conducted: (1) anodal and (2) sham stimulation while the participant is executing the overt speech monitoring task, and (3) anodal and (4) sham stimulation combined with the inner speech monitoring task (Figure 1A). Each stimulation /performance combination will be administrated during one session with an interval of one week between the combinations. Participants will be randomly assigned into one of the four different session orders previously mentioned at study design. For example, the first patient will receive sessions in an ABCD sequence, the second one in BCDA order, and so on (19).
Procedure for the study and each session. A, diagram of the study; after inclusion, each participant completes four different sessions with seven days interval in between. The order of sessions will be randomly selected; B, the Schematic overview of each session; each session consists of three parts: Preparation and practice section (10 minutes), online stimulation (19 minutes SM task under 20 minutes tDCS), and offline stimulation (19 minutes SM task without tDCS). In each session, the task will be assigned to inner or overt speech monitoring, and the stimulation type is real or sham tDCS.
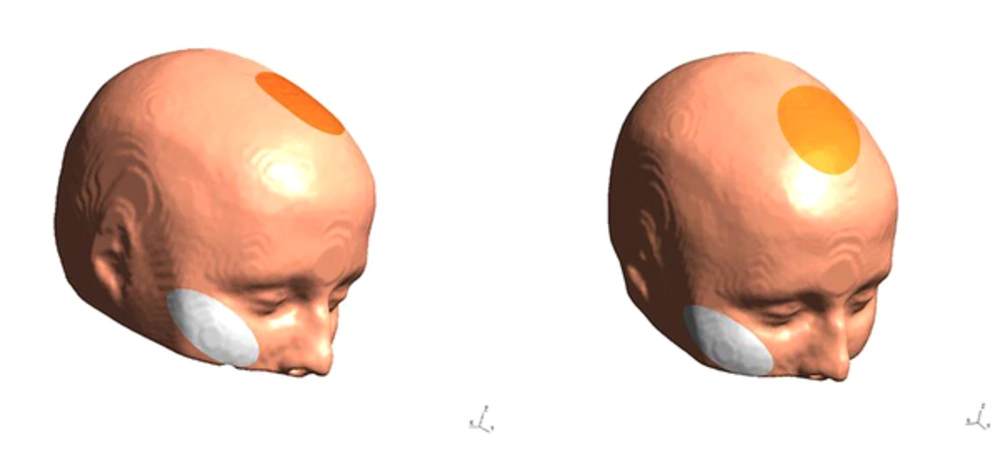
Stimulation will be performed by a tDCS device (Starstim-Neuroelectrics Instrument Controller, version 1.3.12, Rev 2014-07-01, Neurolelectrics, Barcelona, Spain). The stimulation electrode will be covered by a saline-soaked sponge with a surface area of 25 cm2 and is fixed with a cap. In all sessions, the anode is placed over the ACC located under the medial forehead (FCz, position, based on the International 10 - 20 System); the cathode is placed on the right cheek (Figure 2). Stimulation is applied for 20 minutes with 30 seconds ramp up and ramp down, at 2 mA intensity. Sham tDCS follows the same procedure as real tDCS, but stimulation is only applied for 30 seconds, before tDCS is turned off without the patient’s knowledge. Participants and the examiner are blinded to the polarity of stimulation. To blind the examiner, the double-blind stimulation mode in the settings tab of NIC software will be activated.
After stimulation, sensations perceived by the patient (such as pain, warmth, burning, and itchiness) during real or sham tDCS will be studied via the Persian version of the questionnaire introduced by Fertonani et al. (22). The examiner will also ask the patient about the onset time and duration of the sensations.
The steps of each session are as follow: At first, participants sit comfortably at a distance of 60 cm from a 14-inch monitor, prepared for tDCS, and perform a practice section of the task with five sample items. Then, tDCS is started and after one minute, the main experimental task is started. After finalizing tDCS and a six-minute break, the second half of this task is executed. Figure 1B gives an overview of each session.
The examiner will record all responses and writes them on an answer sheet in detail. Response coding will be conducted by two blinded trained experts and the inter-rater correlation will be calculated.
2.7. Outcome Measures
The patients’ performance will be scored during tDCS (online task) and in the first 20 minutes immediately after the end of stimulation (offline), in accordance with previous studies about the time that tDCS effects lasted (13, 23).
The primary outcomes are the dichotomous measure of naming accuracy and monitoring behaviors for each item. Specifically, the first naming attempt will be coded based on its accuracy as a correct response or speech error (phonological and semantic errors, as well as fragments). The following responses are coded as monitoring behaviors. Behaviors such as producing multiple unsuccessful naming responses, expressing inadequacy of responses, and related reactions (e g, target = iron, response = “Water, I don’t know.”) are indicators of response detection. The immediate or delayed successful production of the target name (e g, target = kitchen, response = “Oh that’s chick, chicken, let’s see, living, kitchen.”) is viewed as response correction (2, 9). Finally, responses are categorized into three groups: responses that are (a) detected and corrected, (b) detected but not corrected, and (c) not detected.
Secondary outcome measurement is the percentage change of each primary outcome measure during each session. The formula for this outcome is (24):
Percentage change = [(offline score – online score)/online score] × 100.
2.8. Statistical Analysis
To investigate the effect of repeated stimulation sessions on naming accuracy in addition to response detection or correction as primary outcome measures mixed-effects binary logistic models will be used (9). Several separate models will be run for each of the primary outcomes in each session part, online versus offline performance. In the definition of these models, the target variables are the dichotomous measures of naming accuracy (error vs. correct), response detection (not detected vs. detected), or correction (not corrected vs. corrected). The fixed factors are “tDCS and monitoring channels” each taking two levels, the random intercept is items, and the subject is considered as a nested variable. A multinomial model with a three-level target (predefined response groups) will also be conducted to analyze the effect of tDCS sessions on different detecting/correcting behavior combinations.
These models will be performed using the lme4 package in R statistical software version 3.0.1. The descriptive statistics, model coefficients, Z-tests, significance values, and post hoc test results will be calculated for all primary outcome measures.
To measure differences in the amount of percentage change between sessions, repeated measures ANOVA will be performed if the data have a normal distribution. If the normality assumption is not obtained, data will be analyzed by a non-parametric test, the Friedman test. These tests will be performed with SPSS version 22. P value for all analyses will be 0.05.
3. Discussion
The current study mainly aims at investigating whether anodal DCS over the ACC affects speech monitoring skill in a group of individuals with aphasia. As noted above, a respective effect of ACC stimulation on error monitoring in patients with schizophrenia is available (18), but to the best of authors` knowledge, the current study is the first one exploring the impact of tDCS on the enhancement of speech monitoring in aphasia, especially over the ACC.
In the current study, tDCS will be used in combination with online picture confrontation naming task, which is the most frequently used paradigm in speech production and verbal monitoring studies (2, 9). In this task, the process of choosing the appropriate name to take in response to a given picture may result in the occurrence of conflict between several phonologically- and semantically- related competitors with the target word. The probable conflict is a source of difficulty with naming pictures in all patients with aphasia as well as a demand for more cognitive control in monitoring theories that proposed the role of ACC (16).
According to the results of the current study, differences in the amount of naming accuracy and speech self-monitoring behaviors between the four test sessions are discussed separately. Specifically, speech monitoring under normal auditory feedback (overt speech) will be compared with noised-masked (inner speech) conditions, including the impact of tDCS on these conditions. Generally, a positive effect of anodal stimulation of ACC in comparison with sham tDCS is expected, which was, possibly associated with a higher rate of naming accuracy and response detection. Furthermore, a performance difference between the monitoring performances of individuals with aphasia during inner versus overt speech monitoring tasks is predicted.
Possible implications of the study results are also explained, including adding new data to mechanistic knowledge about the involvement of the ACC in inner and overt speech monitoring. Compatible with traditional treatment methods to improve self-control (8, 10, 11), the use of tDCS over the ACC may be an effective clinical option for the speech self-monitoring treatment in aphasia. Additionally, the effect of the ACC stimulation on naming disorder recovery, which is the most prominent language impairment following post-stroke aphasia will be discussed.
The number of task stimuli under stimulation is one of the limitations of the current study. Regarding the safe duration of active tDCS in the current protocol (20 minutes as the regular duration of each tDCS session with 2 mA intensity), in addition to the suitable time that individuals with aphasia need to name each stimulus and monitor it (at least 20 seconds), increasing number of task items was not feasible. Another anticipated limitation of the pilot study is gathering the suitable sample size, as well as convincing patients and their therapists to substitute the routine speech therapy sessions with those of the current protocol for one month. The absence of electrophysiological measurements to evaluate respective changes of ACC associated with anodal/sham stimulation is also a constraint of the current study.