1. Introduction
Aphasia is an acquired language injury pertaining to brain injury rather than cognitive, motor, or sensory damage. Aphasia can be a common result of stroke in the cortical and subcortical structures of the left hemisphere, which are blood supplied by the middle cerebral artery. Such damage to the brain can affect all language components (phonology, morphology, syntax, semantics, and pragmatics) in all its modalities (speaking, reading, writing, and listening), and the output (speaking) and input (understanding) of the language (1-3). Two-fifths of the individuals that immediately have aphasia after a stroke develop persistent aphasia following a year called chronic aphasia (4). In the acute phase of the stroke, most people show certain degrees of spontaneous recovery, most of which occurs during the first 2 - 3 months (1). Most people with aphasia, however, undergo chronic deficits, which require treatment (1, 2, 5). The mechanisms of neurological recovery in aphasia are still largely unknown, yet significantly depend on the amount of plasticity in the patient's brain after the stroke (1). Contrary to the past myths, the brain is currently recognized as the most dynamic organ of the body. In fact, neuroplasticity enables the brain to repair, modify, and resist damages (6). Neuroplasticity is considered as the capacity of brain to alter at the cellular (neural plasticity) or the behavioral level (behavioral plasticity), which can be either adaptive or incompatible. Brain adaptive plasticity involves efficient redirecting, while incompatible plasticity furthers aphasia symptoms and results in poor recovery due to the brain’s inefficient reprogramming. There are numerous neuroplasticity mechanisms including biochemical, physiological, and structural changes with numerous consequences in behavioral plasticity. Cellular plasticity allows the brain to learn new behaviors, which can in turn alter the brain and further strengthen the behavior. Therefore, plasticity both results from, and entails behavioral changes. Brain injury leads to neurophysiologic changes in the brain that influence behaviors, which in turn, result in more changes in the brain (7-10). There is paucity of evidence regarding the effectiveness of different types of neuroplasticity in improving language in patients with aphasia and the role of the left and right hemispheres with regards to this phenomenon. Old evidence suggests that the role of the right hemisphere homologues region is offset by the lost linguistic performance; novel findings, nonetheless, point to the fact that this hemisphere is ineffective or even incompatible. The current study, therefore, aimed at providing a systematic review of the effectiveness of various types of neuroplasticity and factors affecting the improvement of language recovery in adults with aphasia. Efforts were further made to provide evidence for the role of the left and right hemispheres in the recovery of language following a stroke.
2. Methods
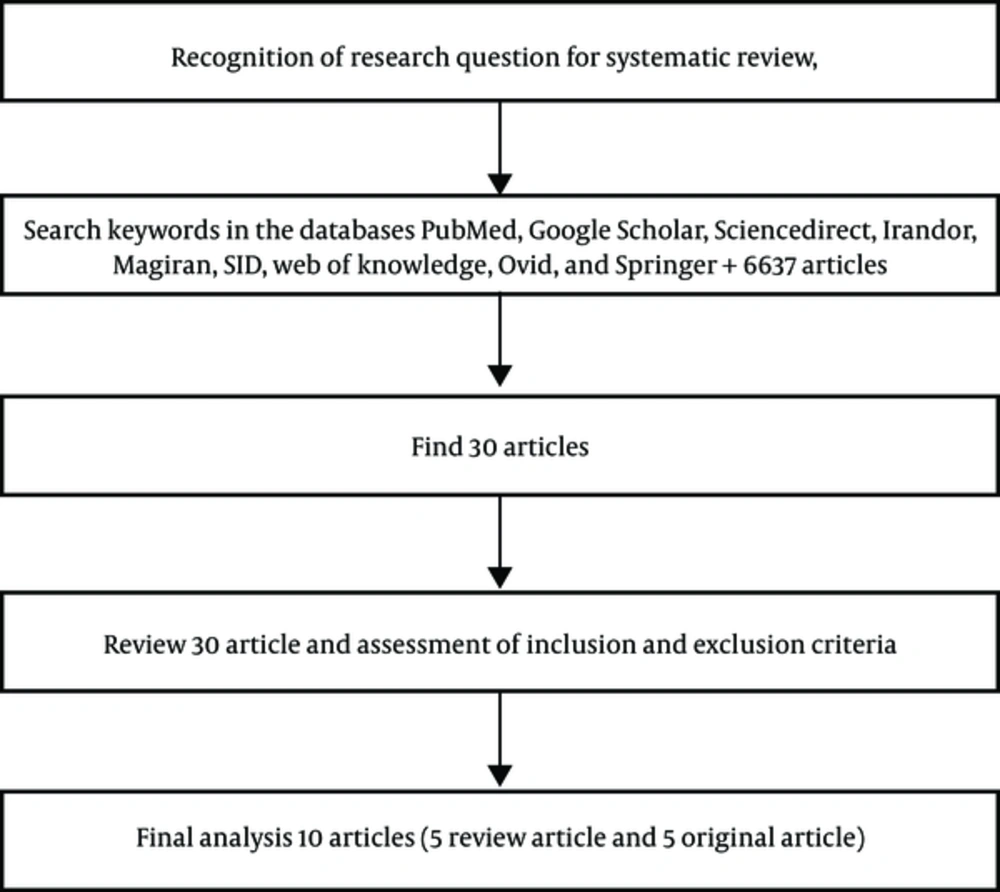
The systematic reviews were conducted by searching all English medical papers registered in the Web of Knowledge, PubMed, Google Scholar, ScienceDirect, Irandoc, Magiran, SID, Ovid, and Springer from January 1990 to April 2017 (latest search belongs to April 30, 2017). Specified in PubMed, the search strategy was "aphasia AND neuronal AND plasticity", through which, papers required for this review were obtained. A reference list of other review papers was used to provide more help. When searching for papers via the mentioned keywords, other related papers suggested by the search engine were also evaluated. The evidence level of each paper was further determined. The PICO format (patient or problem, intervention, comparison, outcome) of this research question is illustrated in Table 1.
| Acronym: Definition | Description |
|---|---|
| P: patients | Adults with acquired aphasia |
| I: intervention | Types of neuroplasticity and its affecting factors that address language recovery |
| C: comparison | Role of the left and right hemispheres in neuroplasticity and language recovery |
| O: outcome | Language recovery defined as a reduction of chronic deficits in all language modalities |
2.1. Inclusion Criteria and Data Extraction
All articles concerning neuroplasticity and aphasia were collected according to the inclusion and exclusion criteria. The included studies (1) employed neuroplasticity and aphasia keywords in their title, (2) revolved around human samples, and (3) adult samples with acquired aphasia (not childhood). The articles excluded from the current study were about non-human samples and the ones examining childhood aphasia and case reports.
Each of the three researchers participating in the current study searched the databases individually. A total of 30 articles were found according to the inclusion criteria. After applying the exclusion criteria, 16 articles were considered as redundant, two were case reports, thus removed; one concerned childhood aphasia and three had no available full-text articles. Three e-mails were sent to the authors of these papers, yet no response was received; hence, they were removed from the systematic review.
Eight articles (three review articles and five original articles) were fully in accordance with the inclusion criteria. The inclusion criteria details are shown in the flowchart (In In addition, according to more detailed studies based on most inclusion criteria, Watila and Balarabe, and Hamilton et al., both review articles, were further added to the list of the selected studies, and 10 articles were reviewed in the end (1, 2).
To assess the scientific level of the evidence, EBMR (evidence-based medicine resources) was employed.
3. Results
Table 2 summarizes the 10 reviewed articles; all comparable in terms of sample size, objectives, and method of investigation. The evidence obtained from the review of these papers indicated that all studies were similar in terms of the three types of changes in the activity of the nervous system following a stroke, which might be closely related to aphasia recovery:
1- Reusing the damaged areas of the left hemisphere and its surroundings in language assignments
2- Acquiring or revealing the ability to process language in the right hemisphere
3- Dysfunctional activation in the right language hemisphere which can prevent language recovery (1).
| Author / Year of Publication | Type and Number of Samples | Study Objectives | Analysis Method | Conclusion |
|---|---|---|---|---|
| Lucchese et al., (11) | 14 aphasia patients (6 females, mean age: 52 yr) | Language performance by intensive speech therapy | Imaging through EEG before and after speech therapy | Intensive speech therapy resulted in language skills improvement |
| Thompson (12) | Review article | Plasticity in language improvement and its related factors | Review of related literature | Parts of the right or left hemisphere or both were used in language improvement |
| Hamilton et al., (1) | Review article | Evidence for a variety of language recovery mechanisms | Review of related literature | Language recovery mechanisms were not incompatible and even may have a hierarchical relationship |
| Marcotte et al., (4) | 9 people with chronic aphasia (5 males, mean age: 62 yr) | Identify the neuroplastic changes associated with recovery from aphasia resulted from the treatment of SFA | fMR imaging before and after treatment of SFA | Better improvement of SFA in chronic aphasia associated with the use of left hemisphere |
| Jacquie et al., (13) | 15 people with chronic aphasia | Changes in behavior and fMRI after two weeks of intensive therapy with PACE and ILATin addition to home practice | fMR imaging before and after treatment | Short-term intensive therapy along with home practice program created sustainable improvement in language |
| Shah et al., (14) | Review article | The effect of Tdcs and Rtms on people with aphasia | Review of related literature | Critical review of the most effective evidence behind the use of these two tools for clinical rehabilitation |
| Marcotte et al., (15) | 9 persons with aphasia (5 males, mean age: 62 yr) and control group of 10 healthy subjects (4 males, mean age: 70 yr) | Changes in the brain network of individuals with aphasia caused by the treatment of SFA | Spatial imaging of the brain in the beginning and at the end of treatment by fMRI | Integration of posterior areas networks involved in language improvement with insignificant relationship |
| Watila and Balarabe (2) | Review article | Factors affecting recovery from aphasia after a stroke | Review of related literature | Aphasia recovery is difficult to predict, but the most powerful predictor are the lesion-related factors |
| Mohr et al., (5) | 14 people with chronic aphasia (5 female, mean age: 56.9 yr) | Study of neurophysiology changes in two groups of aphasia treatment CIAT and ILAT | Through registration of magnetoencephalography | Language functional recovery is associated with neuroplastic changes in both hemispheres |
| Hamilton (16) | Review article | Network changes appear spontaneously and the role of neuroplasticity in the language treatment | Review of related literature | Neuroscience with behavioral perspective to neuroplasticity was removed from the question |
Abbreviations: CIAT: constraint‐induced aphasia therapy, ILAT: intensive language action therapy, rTMS: repetitive transcranial magnetic stimulation, tDCS: transcranial direct current stimulation.
The evidence pertaining to each type of plasticity and their role in language recovery were discussed.
4. Discussion
4.1. The Role of Left Hemisphere in Aphasia Recovery
Significant evidence suggests that the areas around the left hemisphere lesion can acquire language ability weeks and months after the stroke (1). In the acute phase, areas around the lesion and perisylvian of the left hemisphere either have limited activity or are utterly inactive. During the sub-acute phase, the recovery of the linguistic activity of both hemispheres, particularly the homologous Broca’s areas, and the complementary area of the right hemisphere, are highly enhanced. In the chronic phase of aphasia recovery, the left hemisphere regions are reused to certain degrees (14). The brain imaging studies of patients with non-fluent aphasia show that spontaneous linguistic recovery is associated with greater activity in the left hemispheric structures. In patients with fluent aphasia, language retrieval occurs more often if the left temporal linguistic networks are relatively well preserved (1).
The basic mechanisms of the increased activity around the left hemisphere lesion are not completely transparent, yet an effective and important factor may be the damaged cortex release inhibitory input, which leads to increased activity around the cortical regions. In general, unilateral injuries, such as damage to the left hemisphere, which cause aphasia, may lead to non-inhibiting cortical activity in two areas: the cortical areas near the same side of the lesion and the homologous regions on the opposite side of the lesion connected by corpus callosum. Therefore, the disinhibition of focal damage in the left hemisphere can facilitate the activity of areas around the lesion related to linguistic tasks, and ultimately increase the reuse of these areas for language processing (1).
4.2. The Role of the Right Hemisphere in the Recovery of Aphasia
Primary candidates for language retrieval are homologous regions of the right hemisphere, the healthy part of the linguistic network in the left hemisphere or both (12). In fact, most evidence suggests that the activity of areas around the lesion on the same damaged side results in better linguistic recovery; on the other hand, the role of the right hemisphere and its activity during linguistic assignments remains absolutely controversial (1, 16). Some believe that the right hemisphere, particularly the inferior frontal gyrus (4), plays a major role in language improvement (1, 14, 16). Some evidence shows that the right hemisphere activity during the treatment of aphasia is either ineffective or results in a maladaptive strategy of recovery, even inhibiting it (1, 14, 16). Shah et al., showed that the involvement of certain areas in the right hemisphere cannot have destructive effects on language improvement, and only using certain regions of the right hemisphere is an obstacle to recovery (14).
The negative effect of the right hemisphere activity on aphasia's recovery implies the concept of inhibition between the hemispheres. According to this theory, when the brain is unilaterally damaged, the damaged hemisphere loses the ability to maintain a healthy hemisphere
(16); therefore, using the right hemisphere for the language may be facilitated by the non-diffusion nervous inhibitory from the damaged left hemisphere (1).
Moreover, the non-dominant hemisphere releases advanced neural inhibitors on the surrounding areas of the left hemisphere lesion and prevents linguistic activities in these areas (16). According to this theory, the improvement of language in the left hemisphere following stroke entails the inhibition of the linguistic functions of the right hemisphere (1).
Some believe that the right hemisphere plays a major compensatory role in acquiring language abilities in aphasia (16). Research on growth and language compensation in people undergoing hemispherectomy showed that the right hemisphere had linguistic capabilities (1, 12). It was also observed in a study that improved linguistic functions worsen after the initial damage to the left hemisphere, when the Perisylvian structure of the right hemisphere is newly injured (12, 16). Accordingly, it is suggested that the ability to process language in the right hemisphere is homologous to the Perisylvian structures in the left hemisphere, yet it is, more often than not, suppressed by the dominant left hemisphere inhibitors (1). Shah et al., suggested that the homologous linguistic areas in the right hemisphere have a major role in primary linguistic recovery (i e, in acute phase of aphasia) rather than the secondary one (i e, in chronic phase of aphasia); thus, the right hemisphere involvement in language recovery may be transient and regarded as a compensatory mechanism prior to the proper application of the areas around the lesion (14). Others say that the activity of the right hemisphere in a person with aphasia during language assignments is a secondary symptom (related to the chronic phase of aphasia), which neither facilitates nor prevents language retrieval (1).
The use of the right hemisphere for linguistic assignments in severely injured people is conducive to general language retrieval; the linguistic abilities in such patients, however, are likely to be ineffective prior to injury, as the non-dominant right hemisphere may not be intrinsically able to process language, contrary to the left hemisphere, due to genetic backgrounds, growth factors, neuroplastic changes occurring during learning, or a combination of these factors (1). It seems that language retrieval is slightly supported either by the remaining nervous pathways of the areas around the lesion in the affected left hemisphere or by the regions in the right hemisphere, previously part of the semantic network of the language, or by both hemispheres (5).
4.3. Factors and Effective Treatment for Neuroplasticity in Aphasia Recovery
Several factors can be effective concerning language retrieval in aphasia. Internal factors are related to neurophysiological processes and occur during spontaneous recovery including nerve regeneration or sprouting, changing the neurotransmitter release, and maintaining the pre-stroke blood flow. Personal factors are related to the degree of primary aphasia, the size and area of lesion, age, education, gender, motivation, left handedness and environmental factors such as family support (2, 4, 12). Table 3 summarizes the effectiveness of these factors and Table 4 demonstrates the common therapeutic methods and the influence of each on the language recovery in individuals with aphasia.
| Factors | The Type of Effect on Language Recoverya |
|---|---|
| Internal factors | |
| Repair or budding nervous | Effective (12) |
| Changes in release of neurotransmitters | Effective (12, 17) |
| Return CBF and CMR to pre-stroke | Effective (2, 12) |
| Personal factors | |
| Size of lesion | Negative effect (1, 2, 12, 18) |
| Lesion location | Positive impact, while maintaining the superior temporal gyrus (especially the posterior section of it) and basal ganglia healthy (1, 2, 12) |
| Initial severity of aphasia | Negative effect (2) |
| Speech defect | Negative impact on global and anomia aphasia (2) |
| Education | Effectless (2, 12) |
| Age | Weak negative impact (2, 17) |
| Gender | Effectless (2, 12, 19, 20) |
| Motivation | Effective (2, 3, 12) |
| Handedness | Effectless (2, 21, 22) |
| Environment | Effective (2) |
Abbreviations: CBF: cerebral blood flow, CMR: cerebral metabolic rate.
a Type and extent of the impact of all factors are based on most studies, with regardless of the results of limited articles.
5. Evidence Limitations
One of the most important limitations was the unavailability of the full-text of three articles.
6. Conclusion
The review of the literature on neuroplasticity in aphasia recovery led to controversial results. Most studies showed the involvement of areas around the left hemisphere lesion in language improvement, some reported the use of the right hemisphere, especially the lower frontal lobe, and certain studies considered the involvement of both hemispheres to be useful in language recovery. Except for a number of people that had a good recovery, many are left with different degrees of language impairment. The prediction of aphasia recovery is difficult due to the interaction of various factors.