1. Background
In the human brain, the corpus callosum is the main connection between the two brain hemispheres (1) with a key role in motor and sensory cortex connections (2). The alterations in the corpus callosum size have shown in several diseases including bipolar disorder (3), Alzheimer’ disease (4), Leukoaraiosis (5), and Williams syndrome (6). Moreover, morphological changes have been reported in some diseases including dyslexia (7), depression (8), Tourette’s syndrome (9), schizophrenia (10), Down’s syndrome (11), and HIV/AIDS (12). The corpus callosum dimensions are various according to race/ethnicity; therefore, the corpus callosum size measurement and gender-related differences are important in the diagnosis of diseases (13).
Several studies have shown that leukoaraiosis (14), subcortical ischemic vascular dementia (15), cerebral microangiopathy (16), leukoaraiosis and disability (17), lacunar stroke (14), and carotid artery stenosis (18) can cause morphometric alterations in the corpus callosum. There is no documented report of the corpus callosum dimensions in stroke patients in Iran.
2. Objectives
This study was conducted to evaluate the corpus callosum dimensions in Iranian stroke patients compared to a healthy Iranian population.
3. Methods
This case-control study was carried out on 40 right-handed men and women referring to an MRI center in Gorgan city in northern Iran in 2013. The age range of the participants in both case and control groups was 50 - 70 years. Consent forms were signed by all the participants according to the Ethics Committee guidelines.
The participants were divided into case and control groups. The case group included 10 male and 10 female patients with stroke. Stroke is the most rapid neurologic deficit cause by the occlusion of vessels, on extravasation of blood into parenchyma and the neurologic deficit lasts for more than 24 hours. There are various subtypes of deficit, such as less than 24 hours on more than 1 day which makes an obvious neurologic deficit. There are various subtypes of a stroke, some of which are subtle and may last less than one day or create no obvious neurological deficits. In this study, we included patients with obvious neurologic deficits (sensory, motor, visual, etc.) who transferred to the emergency department by their families or pre-hospital medical care staff. The patients experienced a stroke for the first time. The interval from symptom onset to admission was 1 - 3 days. Moreover, the interval from the clinical diagnosis onset to MRI was at least 7 days. We also included 20 subjects in the control group (10 men and 10 women) who had a headache with normal MRI and no specific signs and symptoms.
The measurements of the brain and corpus callosum were done on the MRI unit (Siemens, Symphony, 1.5 Tesla) in the axial and vertical planes using T1- and T2-weighted sequences. The widths of the rostrum, body, and splenium, the anterior to posterior length, and the maximum height of the corpus callosum were measured using a mid-sagittal view of the cerebral hemispheres. One line was first delineated between the inferior borders of the splenium and rostrum and a second line was delineated vertical to the first line to divide the corpus callosum into two sections. The width of the middle portion, which was considered the width of the body of the corpus callosum (B), was dimensioned along a vertical line. The anterior to posterior length and the maximum height of the corpus callosum were denoted as L and H, respectively. The ratios of B/L and B/H were also measured. Student’s unpaired t-test and regression analysis were used for the analysis of data at the significance level of 95%.
4. Results
The mean width ± SD of the rostrum, splenium, and body of the corpus callosum was significantly lower in the stroke patients than in controls (9.84 ± 1.7 vs. 11.20 ± 1.3 mm, P < 0.01; 10.32 ± 1.9 vs. 11.98 ± 0.9 mm, P < 0.002; 6.20 ± 1.0 vs. 6.84 ± 0.6 mm, P < 0.03, respectively) (Table 1). The widths of the rostrum, splenium, and body of the corpus callosum were significantly lower in male stroke patients than in controls (9.45 ± 1.7 vs. 11.65 ± 1.2 mm, P = 0.003; 9.6 ± 1.9 vs. 11.98 ± 0.8 mm, P = 0.003; 5.64 ± 0.9 vs. 6.44 ± 0.7 mm, P = 0.05, respectively) (Table 2). However, these indices were not significantly lower in female stroke patients than in controls (Table 2). Logistic regression showed only was the width of the splenium significantly correlated with stroke (95% CI: 1.22 - 4.51) (Table 3).
| Controlsa (N = 20) | Patientsa (N = 20) | % of Differences | P Valuesb | |
|---|---|---|---|---|
| Rostrum (a) | 11.20 ± 1.3 | 9.84 ± 1.7 | 12.14 | 0.01 |
| Splenium (b) | 11.98 ± 0.9 | 10.32 ± 1.9 | 13.85 | 0.002 |
| Body (c) | 6.84 ± 0.6 | 6.20 ± 1.0 | 9.35 | 0.03 |
| Length (d) | 73.09 ± 4.4 | 73.97 ± 6.0 | +1.2 | NS |
| Height (e) | 26.83 ± 2.8 | 28.52 ± 3.7 | +6.3 | NS |
| Body/length (c/d) | 0.09 ± 0.01 | 0.08 ± 0.01 | 11.11 | NS |
| Body/height (c/e) | 0.25 ± 0.02 | 0.22 ± 0.05 | 12 | NS |
Abbreviation: NS, non-significant.
aValues are expressed as mean ± SD in mm.
bP values compared to control subjects by Student’s t-test.
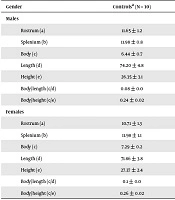
| Gender, Characteristics | Controlsa (N = 10) | Patientsa (N = 10) | % of Differences | P Valuesb |
|---|---|---|---|---|
| Males | ||||
| Rostrum (a) | 11.65 ± 1.2 | 9.45 ± 1.7 | 18.88 | 0.006 |
| Splenium (b) | 11.98 ± 0.8 | 9.60 ± 1.9 | 19.86 | 0.003 |
| Body (c) | 6.44 ± 0.7 | 5.64 ± 0.9 | 12.42 | 0.05 |
| Length (d) | 74.20 ± 4.8 | 74.20 ± 8.0 | 0 | NS |
| Height (e) | 26.35 ± 3.1 | 28.73 ± 5.0 | +9.3 | NS |
| Body/length (c/d) | 0.08 ± 0.0 | 0.07 ± 0.01 | 12.5 | NS |
| Body/height (c/e) | 0.24 ± 0.02 | 0.20 ± 0.06 | 16.66 | NS |
| Females | ||||
| Rostrum (a) | 10.71 ± 1.3 | 10.22 ± 1.6 | 4.57 | NS |
| Splenium (b) | 11.98 ± 1.1 | 11.06 ± 1.6 | 7.68 | NS |
| Body (c) | 7.29 ± 0.2 | 6.76 ± 0.9 | 7.27 | NS |
| Length (d) | 71.86 ± 3.8 | 73.76 ± 3.5 | +2.64 | NS |
| Height (e) | 27.37 ± 2.4 | 28.31 ± 2.2 | +3.43 | NS |
| Body/length (c/d) | 0.1 ± 0.0 | 0.09 ± 0.01 | 10 | NS |
| Body/height (c/e) | 0.26 ± 0.02 | 0.24 ± 0.04 | 7.69 | NS |
Abbreviation: NS, non-significant.
aValues are expressed as mean ± SD in mm.
bP values compared to control subjects by Student’s t-test.
| Controls (N = 20) | Patients (N = 20) | % Of Differences | P Values | |
|---|---|---|---|---|
| Rostrum (a) | 1.80 (1.10 - 2.97) | 0.019 | 1.33 (0.72 - 2.46) | 0.35 |
| Splenium (b) | 2.35 (1.2 - 4.51) | 0.010 | 2.35 (1.22 - 4.51) | 0.01 |
| Body (c) | 2.38 (1.0 - 5.70) | 0.051 | 1.15 (0.35 - 3.76) | 0.81 |
| Length (d) | 0.96 (0.85 - 1.09) | 0.602 | - | - |
| Height (e) | 0.84 (0.68 - 1.05) | 0.138 | - | - |
| Body/length (c/d) | 4.06 (0.01 - 1.02) | 0.069 | - | - |
| Body/height (c/e) | 3.0 (2.19 - 4.11) | 0.051 | - | - |
aLinear regression and multi regression tests.
bSignificant at P < 0.05.
5. Discussion
The results of the current study demonstrated that the size of the corpus callosum was significantly lower in stroke patients than in healthy controls. The atrophy of the corpus callosum can be figured out by MRI. Therefore, in this study, we used MRI for assessing the atrophy of the corpus callosum.
Wu et al. in 2015 reported differences in the size of the corpus callosum between stroke patients and healthy subjects; the size of the corpus callosum was affected by the sex of subjects, as well (15).
Yamauchi et al. study using T2-weighted MRI showed diffuse high-intensity areas in the white matter of the brain hemispheres of patients. Indeed, the callosal area had a significantly smaller size in stroke patients (14). Wittstock et al. study indicated that the callosal function was affected by age-associated cerebral microangiopathy. In addition, they stated that chronic demyelination of callosal fibers may happen in cerebral microangiopathy (16).
Some factors other than white matter damage may contribute to callosal atrophy. The size of the corpus callosum is variable in the general population. In addition, risk factors for stroke may be related to the severity of the corpus callosum atrophy. For example, hypertension is associated with brain atrophy, suggesting that hypertensive patients may have a smaller corpus callosum.
Based on Wu et al. study using fractional anisotropy, the thicknesses of the genu, the anterior third, middle, and posterior third of the body of the corpus callosum, and the splenium of the corpus callosum had smaller sizes in subcortical ischemic vascular dementia patients than in normal subjects. Moreover, they showed lower fractional anisotropy values of the genu and splenium of the corpus callosum in subcortical ischemic vascular dementia patients (15). Cortical reorganization and motor outcome are affected by subcortical stroke through the degeneration of transcallosal fibers connecting sensorimotor regions of the two brain hemispheres (19). Indeed, according to Yamauchi et al. findings, the size of the corpus callosum and age of subjects are significant independent predictors of the scores of the mini-mental state examination in patients with white matter high-intensity lesions (5). Yamauchi et al. study in 2000 reported that cortical neuronal damage in large cerebral arterial occlusive diseases can cause the atrophy of corpus callosum with a reduction in cortical benzodiazepine receptor binding (20).
5.1. Conclusions
This study showed that stroke reduces the morphometric indices of the corpus callosum, particularly in the splenium of men.