1. Background
Over the past 3 decades, most people have been exposed to many new physical and chemical agents. A major physical agent is the electromagnetic field (EMF) that among other agents is widespread and ubiquitous in modern daily life. Therefore, most people are exposed to extremely low frequency electromagnetic field (ELF-EMF) that are emitted by power lines, electrical panels, transformers, domestic electrical, and electronic devices (1-3).
Associations between exposure to ELF-EMF and possible health hazards to mankind have been investigated by several epidemiological and experimental studies (4-8). Most of the epidemiological studies have showed that exposure to ELF-EMF increases the incidence of certain types of cancer, especially acute childhood leukemia (9-11).
Furthermore, several in vitro and in vivo studies have been published on the biological effects of ELF- EMF, and a number of hypothetical mechanisms have been detected (12). A limited number of these studies have demonstrated that ELF-EMF promotes carcinogenic effects. Some available evidence indicates that exposure to ELF-EMF could enhance cell proliferation and inhibit apoptosis (13-15). Several studies indicated that ELF–EMFs exposure has effects on regulation and chromosomal structure (16-18). Moreover, other in vitro studies suggested that ELF-EMF could change the expression of some proteins, which are involved in control of cell proliferation (8, 19) while others demonstrate that exposure to ELF-EMF has no effects on cell proliferation, DNA replication, and regulation (20). The result of these studies varied with different or opposite effects. Some of these conflicting data might be due to differences in frequency, intensity, duration, and also certain types of cell lines (21). Generally, despite of profound studies, there have not been any accepted molecular mechanisms that explain carcinogenic effects of ELF-EMF (22). Proteomics is a powerful technique to elucidate underlying mechanism of exposed cells.
In our previous study, the effect of 3 Hz and 60 Hz of ELF/EMF exposure on male rats was related to anxiety-like behaviors (decrease in locomotor activity and a decrease in unit activity of LC after 2 hours of exposure), long-term memory, and unit activity; it was also found that the electromagnetic fields might alter short term memory and have mild or no effects on long term memory (7). Accordingly, to find the molecular mechanisms behind these behavioral results, this study investigated the possible effects of 50 Hz ELF-EMF, with different intensities (0.5 and 1 mT) on protein expression using proteomics in rat hippocampus.
2. Methods
2.1. Materials
The chemicals were mainly obtained from Sigma-Aldrich (St. Louis, MO, USA) except (DTT), iodoacetamide (IA), Biolytes, Ready Strip™ IPG strips, mineral oil, Criterion precast polyacrylamide gels, urea, dithiothreitol and TGS, and XT MES electrophoresis running buffers, which were obtained from Bio-RAD (Hercules, CA, USA).
2.2. Animals
Adult male Wistar rats (250 to 300 g) were housed 3 to 4 rat/cage in a standard room (constant temperature, humidity, and 12-hour dark-light cycles) and had free access to water and food ad libitum. This study was approved by the ethics committee of Semnan University of Medical Sciences and was performed in accordance with the policies of the guidelines for the care and use of laboratory animals (NIH).
2.3. Exposure System
An ELF/EMF generator calibrated with a digital gauss meter (Magna MG-701, Magna Co. Ltd, Tokyo, Japan) was used to produce the electromagnetic field. It was able to generate homogenous sinusoidal ELF-EMF, with intensity of 0.5 and 1 mT (the occupational magnetic flux density and twice this level) (23) and frequency of 50 Hz, with a 40-cm diameter Helmholtz coil placed around the exposure cage (Figure 1) for 3 hours/day for 2 and 4 weeks. Control rats were housed in their cage outside the coil, while sham exposed rats were maintained for an equal period of time inside the exposure cage with the generator turned off. In all experiments, the room was maintained at a constant room temperature (25 ± 1.0°C).
2.4. Experimental Group
The proteome profile of rat hippocampus was analyzed in 2 main groups. Group 1 contained rats expose to 0.5 mT for 2 weeks (A), 4 weeks (B), and control without radiation (C). Group 2 contained rats exposed to 1 mT for 2 weeks (D), 4 weeks (E), and control (C). In each group, there were sham rats for 2 and 4 weeks.
2.5. Preparation
After exposing different experimental groups to 50 Hz ELF-EMF with different intensities, rats were decapitated and the hippocampus area of the brain was removed and fresh tissue samples of the hippocampus tissue was snap frozen and kept in liquid nitrogen until use. Tissue samples were powdered by a mikro dismembrator at maximum speed for 60 seconds under liquid nitrogen conditions. Each powdered tissue sample was added to an appropriate amount of lysis buffer containing 8 M urea, 4% CHAPS, 40 mM DTT, 2% pharmalyte (pH 3 - 10 NL), 1 mM PMSF, and 1mM EDTA. Samples were sonicated with a probe sonicator for 5 minutes and centrifuged at 40,000 g for 30 minutes at 4°C. Protein concentration in collected supernatants was determined using the Bradford's method. After quantification of proteins, the supernatants were kept at -20°C until use for electrophoresis.
2.6. Two-Dimensional Gel Electrophoresis
In each group, 400 μg of the extracted protein was separately mixed with rehydration buffer and applied to a 17-cm pH 3 to 10, Immobilized pH Gradient (IPG) strip and was passively rehydrated with the above sample solution overnight at room temperature. Isoelectro-focusing (IEF) was performed by increasing the voltage from 500 to 8000 V during the first 3 hours, and then a gradient pattern was used to achieve 8000 V for 3 hours. Following the IEF, IPG strips were incubated in equilibration buffer containing 6 M urea, 30% glycerol, 2% SDS, 2% DTT and then alkylated for 20 minutes in the same buffer with 2.5% iodoacetamide instead of DTT, to separate the second dimension; the treated strips were transferred onto 12% SDS-polyacrylamide slab gel and sealed with 1% agarose. The gels were run with 2.5 W each for 30 minutes and 15 W each until the front of bromophenol blue reached the end of the gel. Analytical gels were stained with Coomassie brilliant blue staining. Gels were scanned using the Bio Rad Image Scanner, and spot detection, matching, and quantitative gel analyses were carried out with the Nonlinear Progenesis software.
2.7. Protein Identification by MALDI-TOF/TOF
In-gel protein digestion was performed with some minor modifications according to the protocol provided by Zhou et al. (24). The data searching was performed on the GPS Explorer (Ver. 3.6, AB SCIEX) using the Mascot search engine (Ver. 2.2, Matrix Science, London, UK), and the international protein index rat database (39871sequences, http://www.ebi.ac.uk/IPI) was used for peptide and protein identification. General protein identification was based on two or more peptides whose ion scores (IS) outmatched a statistical threshold (P < 0.05).
2.8. Statistical Analysis
Two-DE gels were scanned and analyzed using the Non-linear Progenesis Same Spot software to compare gels and spots together. In order to perform multiple comparisons between experimental groups, analysis of variance (ANOVAs) was used (P < 0.05) and all the data were presented as means ± SE. Other multivariate analyses on protein expressions involved hierarchical clustering and principal components analysis.
2.9. Bioinformatics
To find out the predicted interactions within the proteins, the identified proteins were used. The well-known “String” online database (http://string-db.org) was used to obtain a functional protein association network for each entry.
The Cytoscape software was used for network construction and structural analysis, which determined the hub protein in network after all the recognized proteins were in accordance to particular processes or functions by questing GO in CluGO/Clupedia.
3. Results
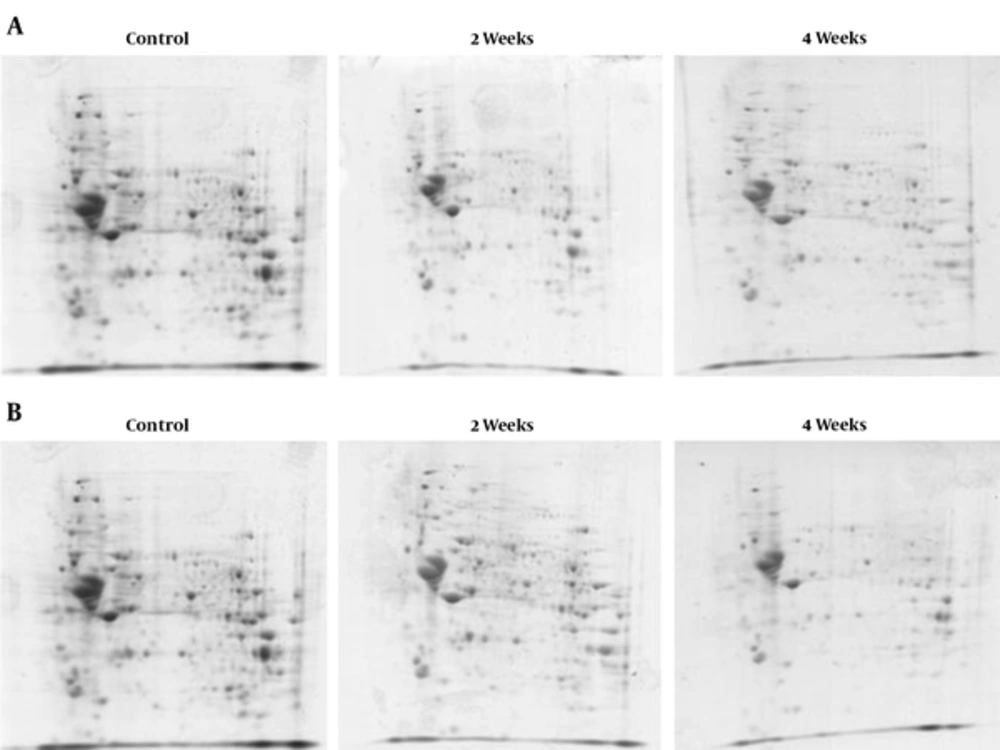
To demonstrate that rat brain cells could be altered with the exposure of 50 Hz ELF-EMF with different intensities (0.5 and 1 mT) for 2 and 4 weeks, the proteome profile of rat hippocampus was determined in 2 different groups. Group 1 contained a protein profile of rats exposed to 0.5 mT for 2 and 4 weeks, and control (Figure 2A). Group 2 contained the protein profile of rats exposed to 1 mT for 2 and 4 weeks and control (Figure 2B). There were sham rats in every group for 2 and 4 weeks, yet because of no difference in protein profile compared to the control, they were deleted from the experimental groups.
The protein patterns of these gels were analyzed using the Progenesis Same Spots software. By using this software, control gels were compared with ELF-EMF exposed gels. The results showed that spots had a statistically significant variation with relative abundance (P < 0.05). Comparison of protein patterns in exposed (50 Hz, 0.5 mT for 2 and 4 weeks) gels with control gels showed that 245 spots had a significant variation. Overall, 64 spots were up-regulated following exposure, while 40 spots were down regulated. Furthermore, 151 significant differences in expressed proteins were identified between exposed (50 Hz, 1 mT for 2 and 4 weeks) and unexposed cells (P ≤ 0.05), amongst which 86 spots were up-regulated and 65 spots were down-regulated.
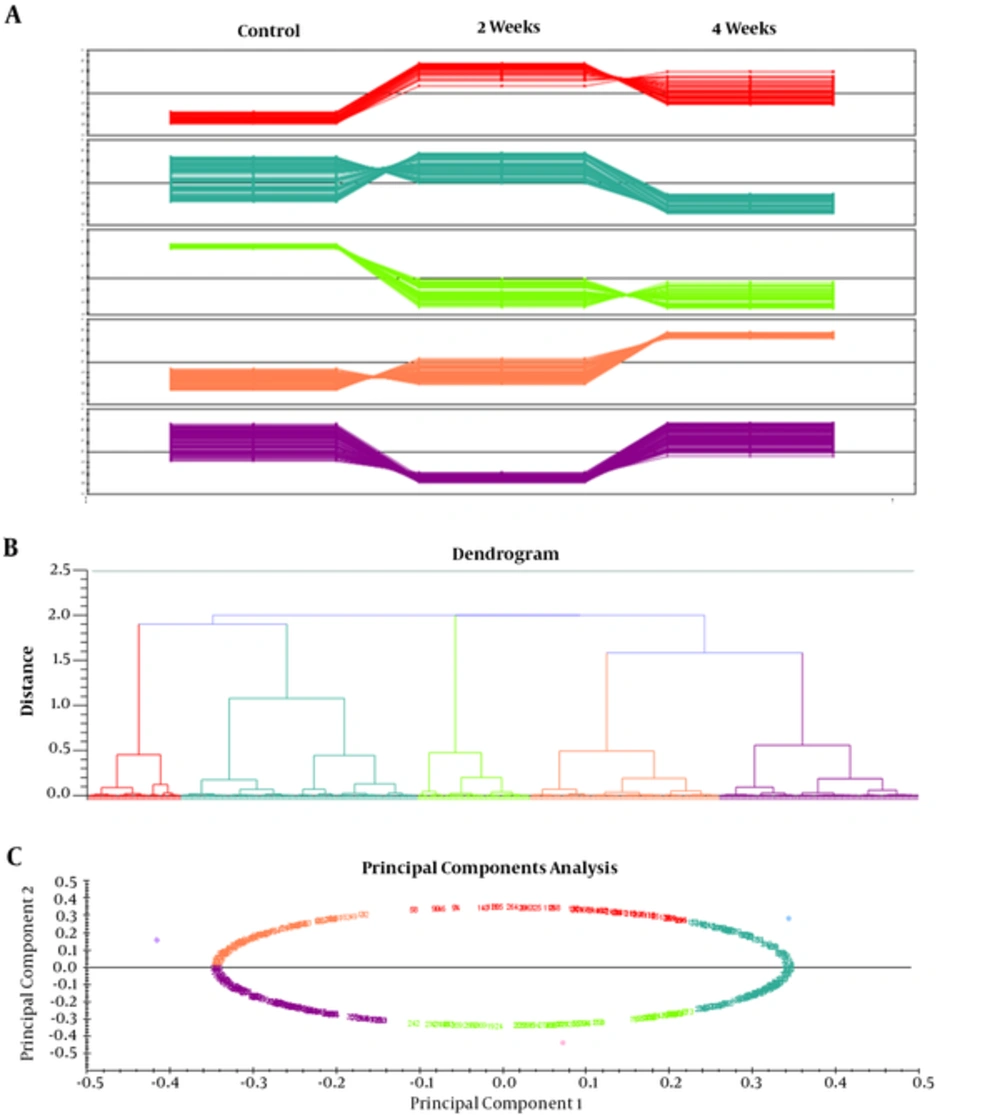
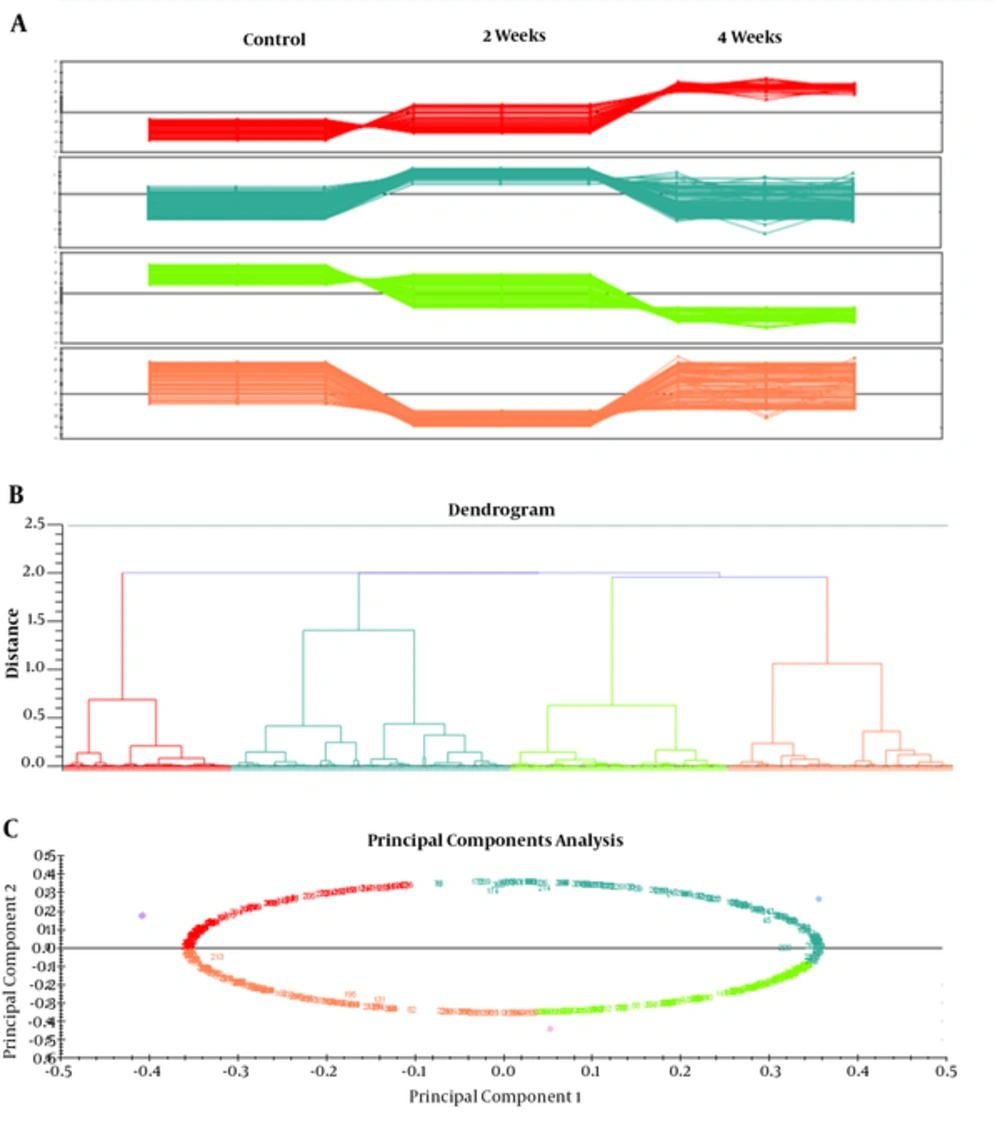
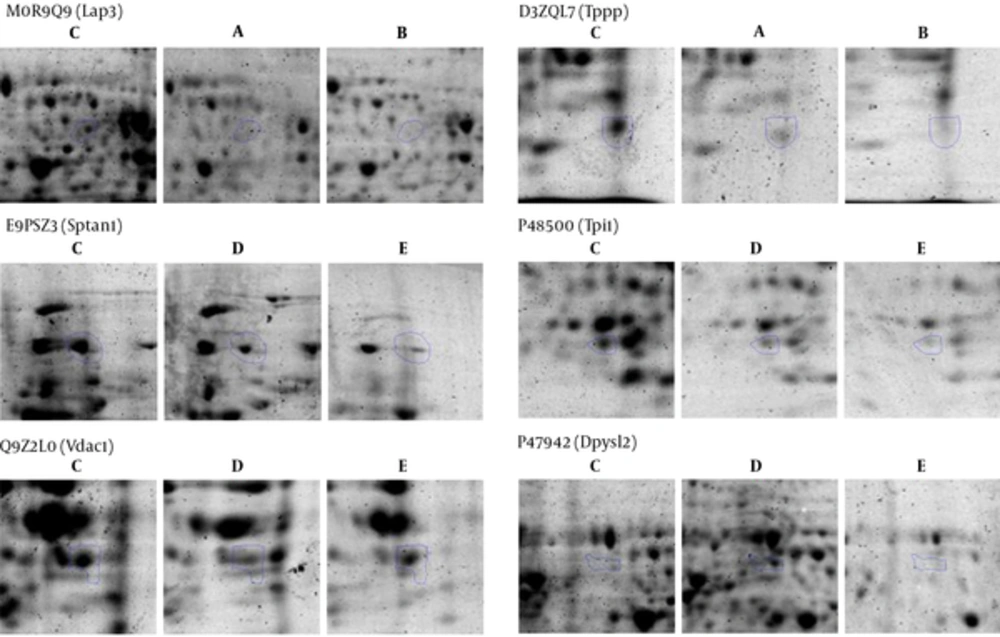
Differences in spot abundance were determined by the Progenesis Same Spot software. Figure 3 represents the protein profile of group 1 based on hierarchical clustering of protein expression pattern that demonstrated 5 clusters that were approved by principal components analysis. Result of PCA also determined that there were no outliers in data. Figure 4 also represents the same analysis in group 2. Significant alteration spots in a number of proteins that were MS-identified are represented in Figure 5. Almost all spots were down-regulated by increasing either intensity of ELF-EMF or the time. The name and ID of this protein is represented in Table 1.
| Protein Name | Uniprot Name | Gene Symbol Name | Description |
|---|---|---|---|
| Spectrin alpha chain | E9PSZ3 | Sptan1 | Non-erythrocytic 1; Fodrin |
| Dihydropyrimidinase-related protein 2 | P47942 | Dpysl2 | Plays a role in neuronal development and polarity |
| Triosephosphateisomerase | P48500 | Tpi1 | Triose phosphate isomerase, abundant glycolytic enzyme |
| Cytosol aminopeptidase (Fragment) | M0R9Q9 | Lap3 | Presumably involved in the processing and regular turnover of intracellular proteins |
| Voltage-dependent anion-selective channel protein 1 | Q9Z2L0 | Vdac1 | Forms a channel through the mitochondrial outer membrane and also the plasma membrane |
| Protein Tppp | D3ZQL7 | Tppp | Tubulin polymerization promoting protein |
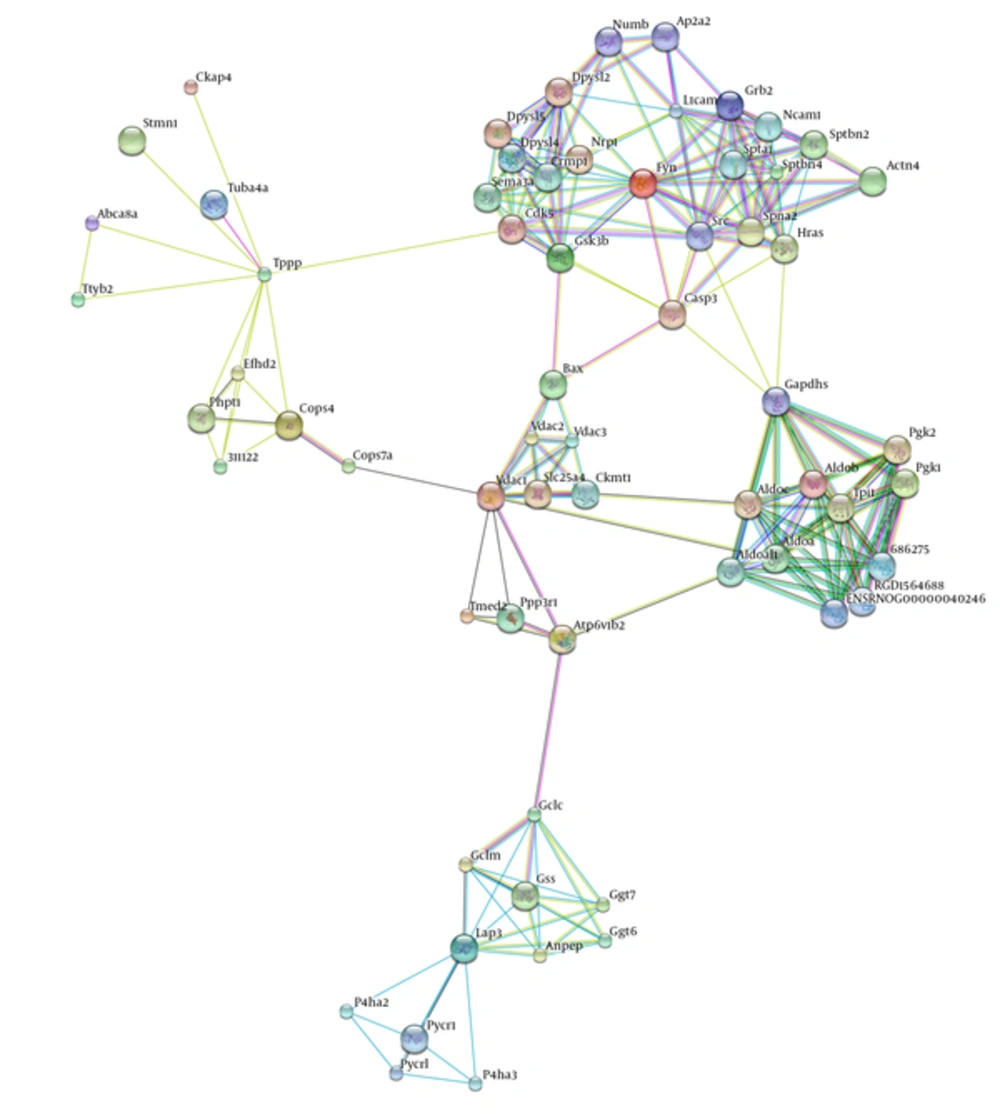
For further analysis, network modeling was used that constructed the protein-protein interaction network with identified proteins. According to Figure 6, by expanding the network constructed, a network with 64 nodes and 224 edges was achieved. Network analysis determined protein hubs that contained all 6 proteins in Table 1 except Sptan1.
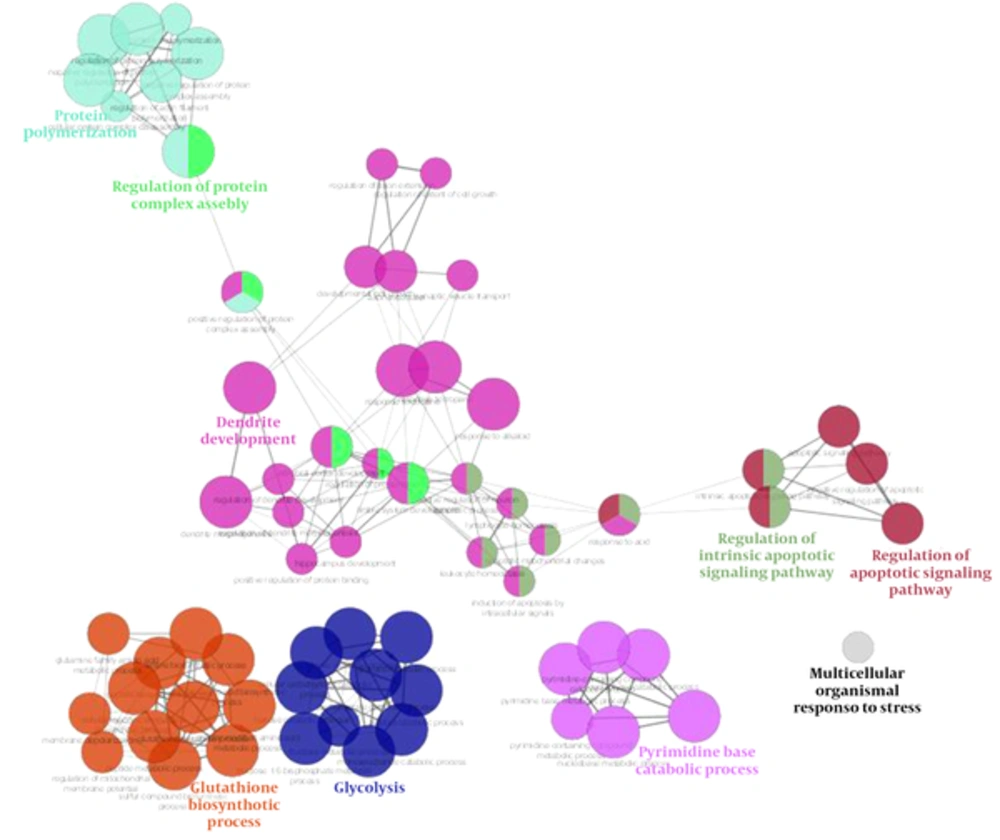
Gene ontology analysis based on Clupedia/CluGO compairing identified proteins that represented biological process categories related to regulation of apoptosis signaling pathway, response to stress, and a number of metabolic processes that represented in Figure 7.
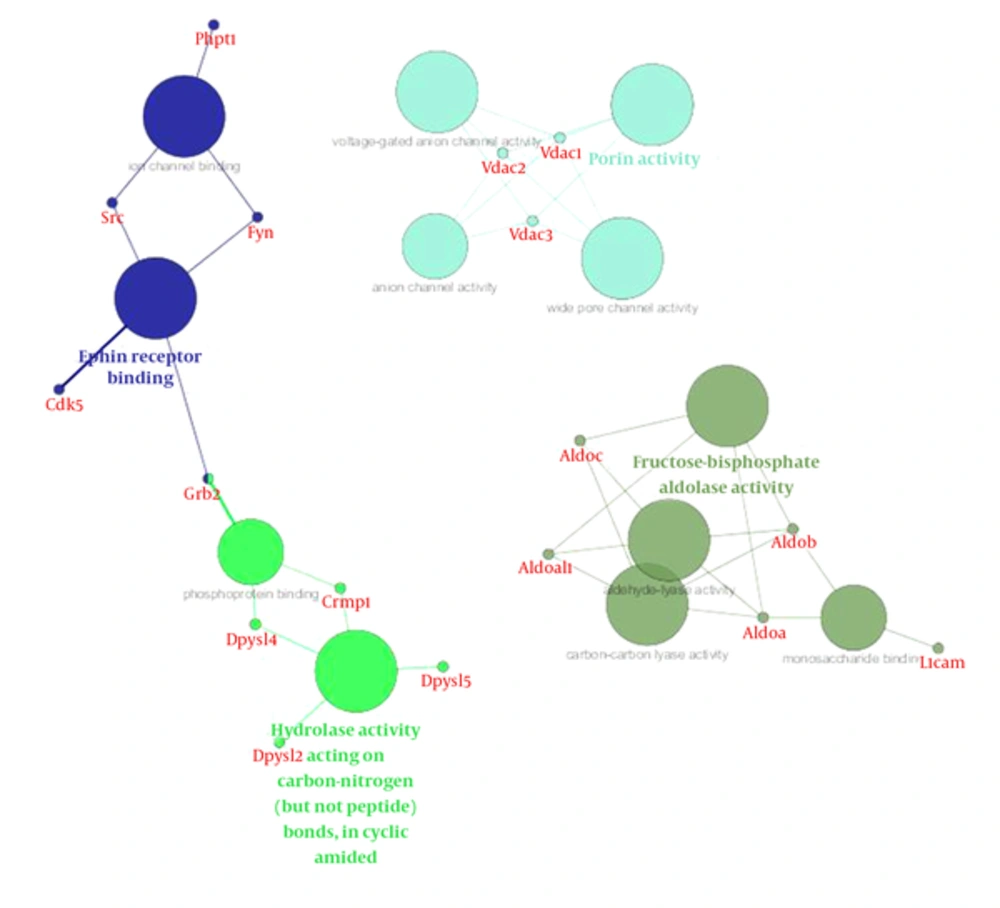
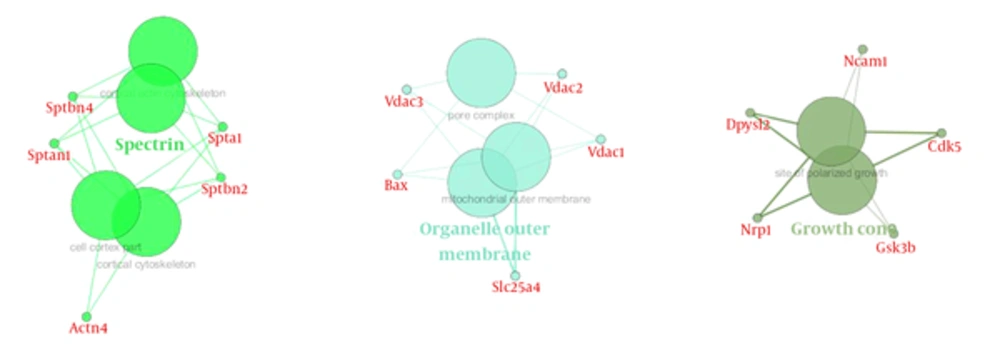
The most important molecular function represented in Figure 8 was related to brain function. The majority of proteins in cellular components are represented in theouter membrane, extracellular protein, and growth cones (Figure 9 and Table 2).
| Name | Degree |
|---|---|
| Fyn | 18 |
| Src | 16 |
| L1cam | 11 |
| Grb2 | 11 |
| Dpysl2 | 11 |
| Aldoa | 11 |
| Cdk5 | 11 |
| Spna2 | 11 |
| Aldoc | 10 |
| Tppp | 10 |
| Hras | 10 |
| Gapdhs | 10 |
| Lap3 | 10 |
| Sema3a | 10 |
| Vdac1 | 10 |
| Gsk3b | 10 |
| Tpi1 | 10 |
| Sptbn2 | 10 |
| Spta1 | 9 |
4. Discussion
The present study investigated the potential effect of exposure to ELF-EMF with different intensities on rat hippocampus and relevant proteome profiles. Our previous study revealed that SHSY5Y cell exposure to 50 Hz ELF-EMF could affect cell morphology and increase the intensity causes a complete decrease in cell proliferation. In our recent study, male rats were exposed to 3 Hz and 60 Hz of extremely low frequency electromagnetic fields and their anxiety-like behaviors, memory retention of passive avoidance, and electrophysiological properties was studied (7). Therefore, the current study investigated the molecular changes in the hippocampus to find the mechanism of this effect. Moreover, the study determined possible changes in the expression levels of proteins at two intensities (0.5 and 1 mT) of 50 Hz ELF-EMF. The data indicates that in the majority of proteins, expression changed with increasing intensity. Here, by proteomic analysis, the study determined different proteome profiles in the hippocampus of rat that are exposed to 0.5 and 1 mT in 2 and 4 weeks. However, these variations in 1mT in the time interval were less than 0.05 mT, because there are 4 clusters for 1 mT encounter and 5 clusters for 0.05 mT. Between the proteins identified, Sptan1 and Dpysl2 were the proteins that were extremely down-regulated in 0.5 mT at both time intervals. Sptan1, spectrin, seems to be involved in secretion, stabilization of plasma membrane and masculinization (25), thus the down regulation of this protein could be directly related to deficiency in movement of rats in behavioral tests. Dpysl2, multifunctional protein of the nervous system, plays a role in neuronal development and polarity, as well as in neuron projection morphogenesis, necessary for signaling-related remodeling of the cytoskeleton (26). Dpysl2 is related to schizophrenia disorder (27). Over-expression of the Dpysl2 causes an increase in the Ca2+ channel density, and CaV2.2 channels have shown a significant increase in vesicular release due to a depolarizing stimulation. The depolarization results in an increased release of glutamate (28). Duan Y et al. have been determined that ELF-EMF causes cognitive impairment associated with alteration of the glutamate level in mice hippocampus so that the molecular mechanisms of neuronal damage attributed to an excessive release of glutamate (29). Therefore, Dpysl2 up regulation in 1 mT after 2 weeks might lead to polarization and depolarization in cells to elicit glutamate. Dpysl2 down regulation after 4 weeks, which is similar to normal conditions, might represent an adaptation system.
The next two proteins are Tpi1 and Lap3, the expressions of which are decreasing through time. Tpi1, Triose phosphate isomerase, abundant glycolytic enzyme coupled with neurodegeneration (30). TPI deficiency is coupled with hemolytic anemia and neurological disorder, which lead to death in early childhood. Mutation and/or posttranslational modification could lead to the formation of an aggregation-prone protein, a characteristic of conformational disorders (31). Therefore, the altered short term memory in rat exposed to electromagnetic fields might be related the decrease of TPI expression.
Lap3 is presumably involved in the processing and regular turnover of intracellular proteins. It seems that Lap3 has no expression in neuropathological conditions as Farrah D et al. revealed no gene expression of Lap3 in Alzheimer’s disease (32). Lap 3 catalyzes the removal of unsubstituted N-terminal amino acids from various peptides. Other proteins had similar expressions between control and 1 mT after 4 weeks, including Vdac1. It has been reported that normal mitochondrial permeability is essential for learning and memory tasks, in which related proteins VDAC isoform as proteins in the mitochondrial outer membrane permeability that increment leads to developmental abnormalities in neuronal architecture. The contributions of the VDAC1 and VDAC3 isoforms in learning and synaptic plasticity have also been determined (33). Herein the down-regulation of VDAC1 after 2 week and up-regulation after 4 weeks might be linked to altered short-term memory and adaptation.
Tppp, tubulin polymerization promoting protein, had a decreased rate in time by 1mT, and may play a role in the polymerization ultrastructure, thus maintaining the integrity of the microtubule network in the brain. TPPP is involved in the normal functions of key proteins i.e. PrP and play a role as protective factors for cells against the damage effects of the accumulation of abnormal forms (24, 34). This protein is directly linked to memory associated hippocampus structure, thus decreasing memory in the exposure radiation might be related to down regulated Tppp expression. VDAC and Tppp rely on cell adaptation in exposure radiation. Network analysis emphasizes the crucial function of these proteins in the brain because structural analysis determined that all these proteins are hub. Gene ontology analysis also determined the functions of these proteins were related to two important brain functions, including cytoskeletal neurons and metabolic processes. These processes were related to short memory formation in the hippocampus so the molecular deficiency in this part of the brain led to a neuronal disorder, such as a neurodegenerative disease (35, 36).
In conclusion, the result of our previous study on behavioral changes (decreased memory and movement defects) affected by increased intensity of ELF-EMF and the findings of the current study showed that adaptation timing in molecular levels were related to down-regulation in critical hippocampus protein expressions, most of which belonged to cytoskeletal processes. Therefore, a disorder in behavioral tests might be linked to disarrangement in hippocampus cell structures.