1. Background
A link between bone loss or bone mineral density and neurodegenerative disease such as Alzheimer’s and Parkinson`s diseases has been recently demonstrated (1, 2). Bone health is an important factor in neurodegenerative diseases given a higher risk of falls and increased incidence of fractures in individuals with neurodegenerative diseases compared to cognitively healthy older adults (3).
Bone tissue is liable for the form and mechanical backing of the body (4). In addition, bones act in the body mineral homeostasis and may regulate the phosphate level of metabolic processes. An important challenge in the area of orthopedic is bone tissue regeneration (5). Since the bone tissues are usually exposed to different damages, such as fractures, they are possible in several ways, such as trauma, osteoarthritis, neoplasm, osteoporosis, congenital defects, etc. (6). Many kinds of treatment options are existing for managing the skeletal losses of bone defects, like bone graft. However, lack of enough donors and the possibility of transmitting diseases are the major drawbacks of auto graft and allograft techniques (7).
It is essential to find novel solutions, through bone tissue engineering (TE), in order to overcome the restrictions of prevalent bone grafting methods. Bone TE approaches are hopefull strategies for healing bone losses and restoring bone defects (8).
Bone TE approaches include the mixture of cells, scaffolds, and conditioned culture situation incorporating biochemical motives to encourage in vitro bone forming (9). Many origins of cells are existing as beneficial candidates in the bone TE field, like hematopoietic stem cells (HSC), mesenchymal stem cells (MSCs), embryonic stem cells (ES), and human endometrial stem cells (hEnSCs) as multipotent cells (10). Endometrial stem cells have been used in this study due to their angiogenesis property, their capacity to differentiate into endoderm-mesoderm and ectoderm, maintaining normal karyotype after 34 consecutive passages, rapid proliferation and their accessibility (11). Constructing Scaffolds that have the ability to form tissues within the body upon transplantation is one of the important elements in bone TE (12). Composite scaffolds, made of polymers and bioactive ceramics, are widely applied in bone TE due to their superior properties (13, 14). Poly L-lactide-co-glycolic (PLGA) is one of the synthetic polymers that has been used in clinics due to its good biocompatibility, controllable degradability and relatively good process ability (15, 16). It was shown that, bioactive glass increase cohesion, development and osteogenic differentiation of stem cells (17, 18). Combining polymers and bioactive glass, in order to obtain new composites, is a novel strategy in bone TE and the composites benefits, including enhancement of mechanical indices, enable better adhesion with bone tissue and bioactivity that progress new bone formation (19-21). There are several methods for synthesizing scaffolds. Electro spinning, is an easy and fast developing method, and prepares a straightforward approach to make long fibers of their diameters ranging from submicron to nanometers (22, 23). Freeze-drying has appeared as a drying process for changing the solution forms of labile materials into sufficiently stable solids in order to be distributed and stored for various applications (24). The aim of this study was to differentiate these cells into osteoblast cells and to culture the differentiated cells onto the scaffolds made of PLGA/Bioglass in order to be used as a new approach for bone regeneration in neurodegenerative disease.
2. Methods
2.1. EnSCs Differentiation into Osteoblast Cells
2.1.1. hEnSCs Isolation and Cultivation
Human endometrial stem cells were gained according to the protocol previously described by Ebrahimi-Barough et al., (25). The protocol for human endometrial-derived stem cells has been approved by the ethics committee of Tehran University of Medical Sciences. Identified human EnSCs at passage 3 were used for experiments.
2.1.2. Osteogenic differentiation
hEnSCs were cultured in 6-well plates, in Dulbecco’s modified eagle medium DMEM with the density of 2 × 104 cells/mL, containing 10% FBS. The seeded cells were divided into two groups; treatment group, which was treated with 7 - 10 µg/mL dexamethasone, 50 µg/mL L-ascorbic acid-2-phosphates, and 10 mM β-glycerophosphate and a control group, which was not cultured in osteogenic media. The cells were cultured at 37°C, in 5.5% CO2, for 21 days. Every 2 days, culture medium was changed and cell morphology was examined by an inverted microscope.
2.1.3. Alizarin Red Staining
To measure the mineralization of the ECM, Alizarin red staining was used. Briefly, on day 21, the cultured cells were stained with 2% Alizarin red at room temperature for 30 minutes after being fixed in 4% paraformaldehyde (Sigma, USA) for 20 minutes. The results were studied qualitatively regarding the intensity of the staining and the surface of the AR-stained positive areas (i.e. red areas).
2.1.4. Immunocytochemical Analysis (ICC)
After being in culture for 21 days, cells were fixed at 4°C for 20 minutes with 4% paraformaldehyde and then the washing process is done three times with phosphate buffered saline (PBS), each time for 5 minutes. Membrane permeabilization was performed by incubating the cells in PBS/0.2% Triton-X 100 (Sigma-Aldrich) at room temperature for 30 minutes. By incubating the cells in 5% goat serum (PBS/BSA) for 45 minutes, non-specific binding sites were clogged. Cells were then incubated overnight with primary anti-bodies; anti-osteocalcin (mouse anti-human, Santa Cruz, USA) and anti-osteopontin (mouse anti-human, Santa Cruz, USA) at a 1:200 dilution; at 4°C. After removing the excess primary antibodies and performing the washing steps, cells were incubated with rabbit anti mouse IgG-FITC, as the secondary antibody, (Santa Cruz, USA) for 1 hour at 37°C. Excess secondary antibody was picked up and after that, cells were washed with PBS. To stain the nuclei, 4, 6-diamidino-2-phenylindole (DAPI, Sigma, USA) was applied. Cells were visualized finally by using a fluorescence microscope (Olympus BX51, Japan).
2.2. Preparation and Characterization of Nanocomposite Scaffolds
2.2.1. Preparation of Electrospun Scaffolds
To obtain a uniform, beadles, and well defined nanofibrous scaffold, the PLGA/BG electrospun scaffolds were prepared under steady state conditions with the ratio of 20% BG and 80% PLGA. Briefly, the spinning initial solutions were prepared by combining PLGA (MW 80.000 g/Mol, 10% w/v; Sigma-Aldrich, USA) to Hexafluoroisopropanol (HFIP), which was stirred overnight. The next step was to add BG powder to the spinning solution. The solution was stirred again at room temperature for about 2 hours until a homogenous mixture was obtained. The prepared solution was then subjected for electrospinning. This process was carried out using electrospinning (Electroris®, Tehran, Iran). In order to perform electrospinning, needles of the syringe were connected to the positive terminal of a high-voltage power supply (Gamma High Voltage Ormond, Beach, Florida, USA) and the velocity (rpm) of the collecting drum was adjusted at the speed of 400 rpm. The electrospinning began when high electric voltage of 12 - 14 kV was obtained. The solutions were electrospun on the same drum simultaneously for a whole period of 6 hours and the scaffolds were then left to be dried overnight.
2.2.2. Preparation of Freeze-Dried Scaffold
Porous PLGA/BG nanocomposites were fabricated as follows: the first step was preparation of the solution by dissolving PLGA (70%) polymer in 5 mL Dioxan. BG powder was then added to the solution with the ratio of 70/30 × w/w (PLGA/BG). The solution was incubated at -20°C in a freezer and was finally kept at -80°C for 24 hours. Afterwards, scaffolds were dried using freeze-drying at -55°C and the pressure of 0.03 mbar.
2.2.3. Scanning Electron Microscopy (SEM)
SEM (XL 30, and Philips, USA) was employed to investigate the surface morphology and diameter of the obtained nanofibers and pores of the nanocomposite scaffolds at an accelerating voltage of 25.0 kV. A little part of nanocomposite and nanofibrous scaffold samples was sputtered by gold prior for observation.
2.3. Study of Cell Attachment and Differentiation on Scaffolds
2.3.1. Preparation of Scaffolds for Cell Seeding
In order to prepare the scaffolds for seeding the cells, they were cut into a proper size using a punch. The PLGA/BG scaffolds were sterilized using 70% ethanol, washed with PBS three times, and were left to dry in a laminar flow cabin overnight under UV exposure before cell seeding.
2.3.2. Cell Attachment Study on Scaffold
Differentiated osteoblast cells were utilized to measure the in vitro cytocompatibility of the synthesized scaffolds. The cells were seeded in DMEM supplemented with streptomycin/penicillin 100 U/mL (1%) and 10% fetal bovine serum (FBS). Cells were trypsinized (0.05% trypsin/0.53 mM EDTA in 0.1M PBS without calcium or magnesium), centrifuged, and resuspended in complete culture medium. Cells were then seeded at the density of 3 × 105 cells/mL onto each scaffold, which was soaked in the culture medium prior to culturing for 2 hours. Scaffold/cells constructs were incubated in a humidified atmosphere containing 5% CO2 at 37°C for 3 days. The samples were then washed twice with PBS prior, then they were fixed in 4% paraformaldehyde for 40 minutes at room temperature. Post-fixation was done in 1% osmium tetroxide and scaffolds were dehydrated in a graded acetone series solution. Subsequently, the samples were kept in a laminar hood in order to be air-dried and used for SEM observation.
2.3.3. Determination of Cell Viability by MTT Test
Cell viability was measured using 3-(4, 5-dimethylthiazol-2-yl)-2, 5 diphenyltetrazolium bromide (MTT) assay on days 1, 3, and 7 post-culture. The MTT assay was done MTT at a concentration of 0.5 mg/mL in PBS (Sigma, Germany). Approximately, 1 × 104 cells/well were cultured into 96-well plates. Cells in per well were treated with 100 µL of MTT solution for 4 hours in a 5% CO2 incubator at 37°C. The MTT solution was then picked up and 100 µL of dimethyl sulfoxide (DMSO, Sigma, Germany) was increased into per well. The plates were shaken on a shaker for 5 minutes prior to read the absorbance using an ELISA reader at 570 nm. In this study, three blank wells of culture plates were applied as negative controls (tissue culture polystyrene (TPS)).
2.3.4. Cell Proliferation Assay by4’6-Diamidino-2-Phenylindole (DAPI) Staining
DAPI staining was applied to recognize cell attachment. HEnSCswere cultured onto the scaffolds and were kept cultured in DMEM/F12 for 1, 3, or 7 days, depending on the experimental groups. Then the samples were washed twice with PBS before being fixed. The samples were fixed 4% paraformaldehyde. Morphological evaluation, cell attachment, and proliferation were measured by DAPI staining.
2.3.5. qRT-PCR (Real Time-Polymerase Chain Reaction)
To detect the expression of osteoblast-specific genes, like collagenI (COL1, 568 pb), BGLAP, IBSP, RUNX2, GAPDH, Real-time PCR was carried out at day 21 post-induction and one week after culturing the cells onto the above-mentione dscaffolds. Elaborations of the primer utilized for RT-PCR are shown in Table 1. Human EnSCs that were differentiated into Osteoblasts were isolated for total RNA extraction using TRIzol reagent (Gibco, USA). Cells were treated by DNase I, RNase-free kit (Takara, Bio, Inc., Shiga, Japan, 2270A) to raise genomic DNA. A revert aid first strand cDNA synthesis kit (Fermentas, USA, K1632) was applied to synthesized complementary DNA. Relative gene expression was measured real-time PCR. A total of 96-well optical reaction plates were used to perform the experiment in a 7500 real-time PCR system (Applied Biosystems, USA). The reaction mixture consisted of 12 µL SYBR® Green PCR Master Mix (Applied Biosystems, USA), 12 ng of cDNA and 1 µL of both forward and reverse primers and the total volume was 20 µL. Annealing temperature of all the primers used in this experiment were the same, which was 60°C. The comparative 2-ΔΔCT method was used for relative gene expression analysis. whole Ct values were normalized to internal control (GAPDH). The relative gene expression amount were presented as the mean of three kinds of experiments.
| Gene | Length, bp | Primer Sequence (5’ - 3’) | Annealing, °C |
|---|---|---|---|
| COL1 | 20 | F ATGGCTGCACGAGTCACACC | 60 |
| 20 | R CAACGTCGAAGCCGAATTCC | ||
| BGLAP | 21 | F GGTGCAGCCTTTGTGTCCAAG | 60 |
| 23 | R AACTCGTCACAGTCCGGATTGAG | ||
| IBSP | 24 | F GATTTCCAGTTCAGGGCAGTAGTG | 60 |
| 27 | R GTTTTCTCCTTCATTTGAAGTCTCCTC | ||
| RUNX2 | 22 | F ACTCTACCACCCCGCTGTCTTC | 60 |
| 21 | R AGTTCTGAAGCACCTGCCTGG | ||
| GAPDH | 15 | F TCGCCAGCCGAGCCA | 60 |
| 20 | R CCTTGACGGTGCCATGGAAT |
2.4. Statistical Analysis
Whole quantitative data were analyzed by employing instate software. All data were presented as mean ± standard deviation (SD) and they were considered significant for P values smaller than 0.05. ANOVA was used for statistical comparisons.
3. Results
3.1. Isolation and Characterization of Human EnSCs
hEnSCs could be separated simply based on their feature of attaching to the plastic flask. A total of 10 days after being in culture, the isolated cells appeared as several clusters that could be used afterwards for sub-culture. After 3 - 4 passages, the morphology of hEnSCs was comparatively protracted and they became comparatively homogeneous. Flow cytometry analysis has been published in previous reports (8). It has been shown that CD90+ (80%), CD105 + (79%), and CD146 + (97%) were highly expressed in endometrial stem cells while expression levels of CD31, CD34, CD133, and CD45 were extremely low.
3.2. Differentiation of hEnscs into Osteoblast Cells
3.2.1. hEnscs Cell Culture
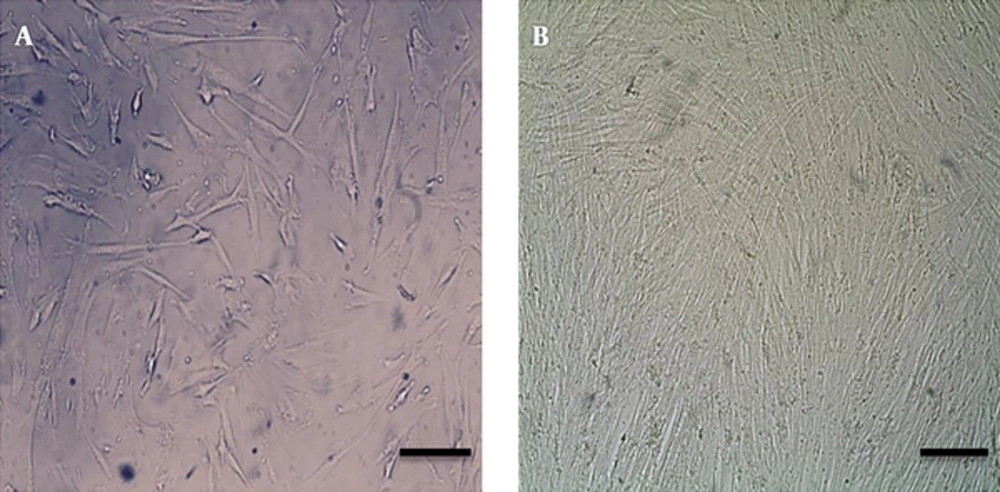
hEnscs primary cultures included cells, which predominantly had fibroblastic shape. This morphology was held throughout the sub-cultures (Figure 1A). Human EnSCs were cultured at a density of 2 × 104 cells/mL in per well of 6-well plates containing osteogenic media and a control group was cultured in plates without osteogenic media for 21 days. The cultured cells were visualized under a microscope during the 21 days of culture (Figure 1B). Differentiation of the human Enscs into osteoblast like cells was analyzed after being in culture for 21 days.
Image of differentiated cells since 21days post induction. A, morphological variations of human endometrial stem cells. hEnSCs after passage3 showed spindle-shape morphologies, similar to fibroblasts. B, 21 days after osteogenic exposing cells that converted to osteoblast like cells. Scale bar = 100 µm.
3.2.2. Alizarin Red Staining
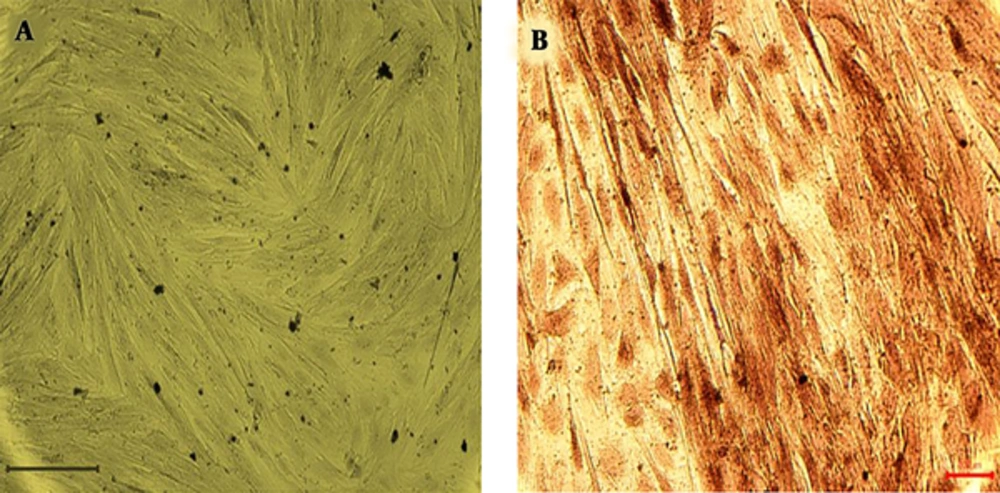
Differentiated seeded cells stained by alizarin red 21 days after treatment. As it can be seen in Figure 2 dark red staining of calcium evidence were seen in cells after disposal to osteogenic media. In the control group (non-differentiated hEnSCs), Alizarin red staining was negative (Figure 2).
3.2.3. Immunocytochemistry of Human Endometrial Stem Cell-Derived Osteoblast-Like Cells
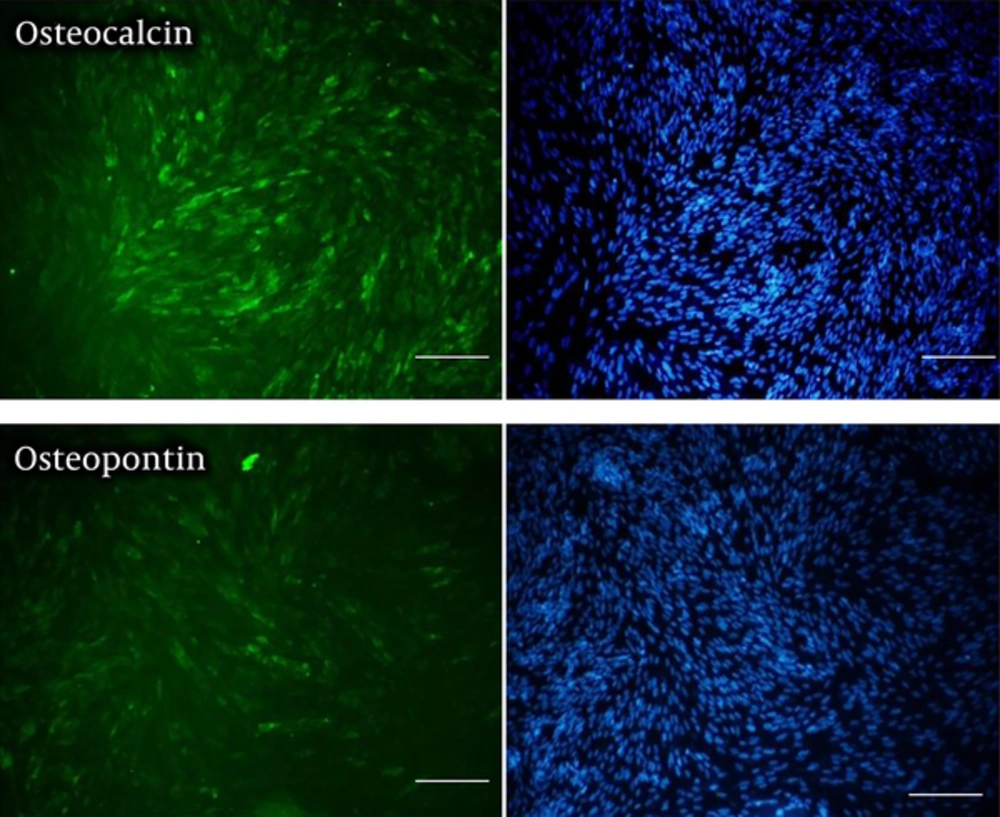
Immunocytochemistry has been done using antibodies specific to osteoblast cell markers, include osteocalcin and osteopontin as seen in Figure 3. The osteoblast cells were derived from Enscs seeded in osteogenic media for 21 days. The non-treated cells for steogenic differentiation were used as the control group. No positive result was observed in the control group and expression of the osteopontin and osteocalcin were positive in the treatment group (Figure 3).
3.3. Characterization of Nanocomposite Scaffolds
3.3.1. Scanning Electron Microscopy (SEM)
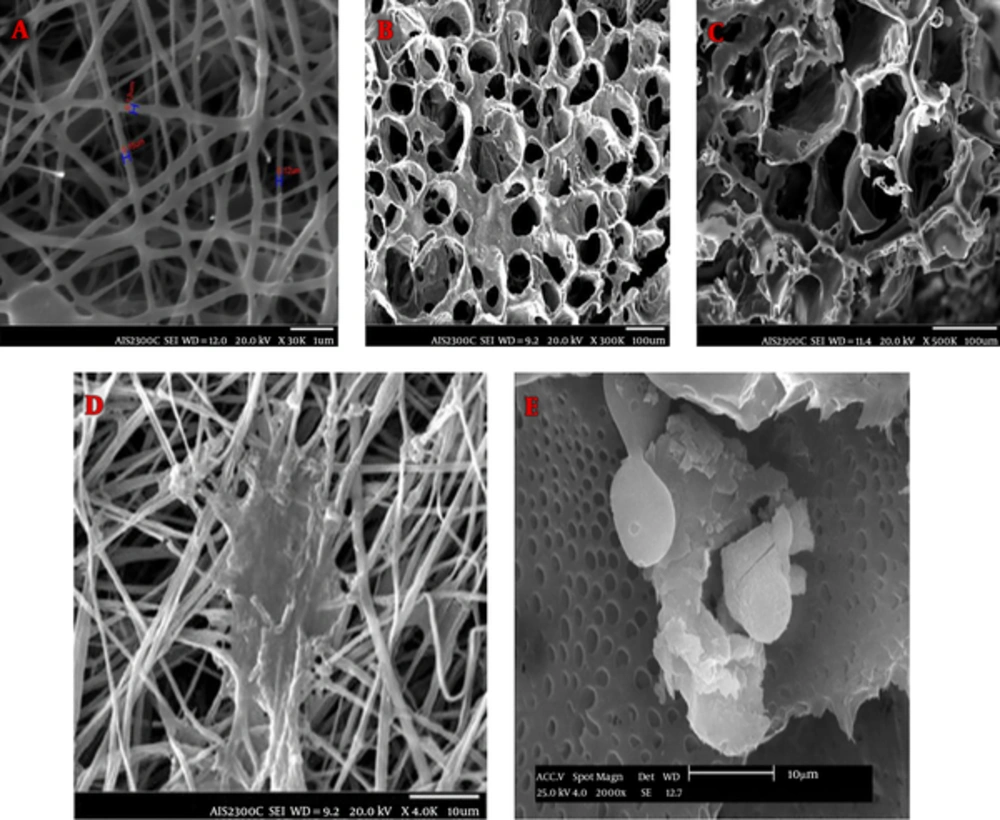
To determine the morphology of the nanocomposites, SEM was perfomed. The pictures taken from the surfaces of the scaffolds by using SEM are shown in Figure 4A, B, and C. A mesh of interconnected pores with almost uniform honeycomb-like shape was seen in Figure 4. The average of these scaffold pore diameters is 177.54 µm (Figure 4), which is appropriate for osteoblast cell’s growth. SEM images illustrated that PLGA/BG nanofibers were bead free and smooth with no branching. The median fiber diameter was 0.14 µu (Figure 4) and the microscopic images showed that the average fiber diameter was in the range of the extracellular matrix protein components. Due to the increased surface to volume at the nanoscale, nano-fibrous scaffolds are more suitable for cell adhesion compared to nanocomposite scaffolds (Figure 4).
3.3.2. Cell Attachment Study on Scaffold Using SEM
SEM micrographs of cell interaction with both PLGA/BG freeze-dried nanocomposite and PLGA/BG electrospun nanofibrous scaffolds, after being cultured for 3 days, are shown in Figure 4. It was observed that cells were adhered and penetrated into the porosity of the scaffolds. Cultured cells were attached to the surface of electrospining scaffold and penetrated into the deep pores of the freeze-dried scaffolds.
SEM micrographs showed that cells were well-joined and cells grew upon both of the scaffolds. Although, more cell attachment and density were foud on PLGA/Bioglass electrospun nanofibrous scaffolds compared to PLGA/Bioglass freeze-dried nanocomposite.
SEM was employed to determine the morphology of the scaffolds. The images taken from the surfaces of the porous nanocomposite scaffolds using SEM are presented in Figure 4.
3.3.3. MTT Assay
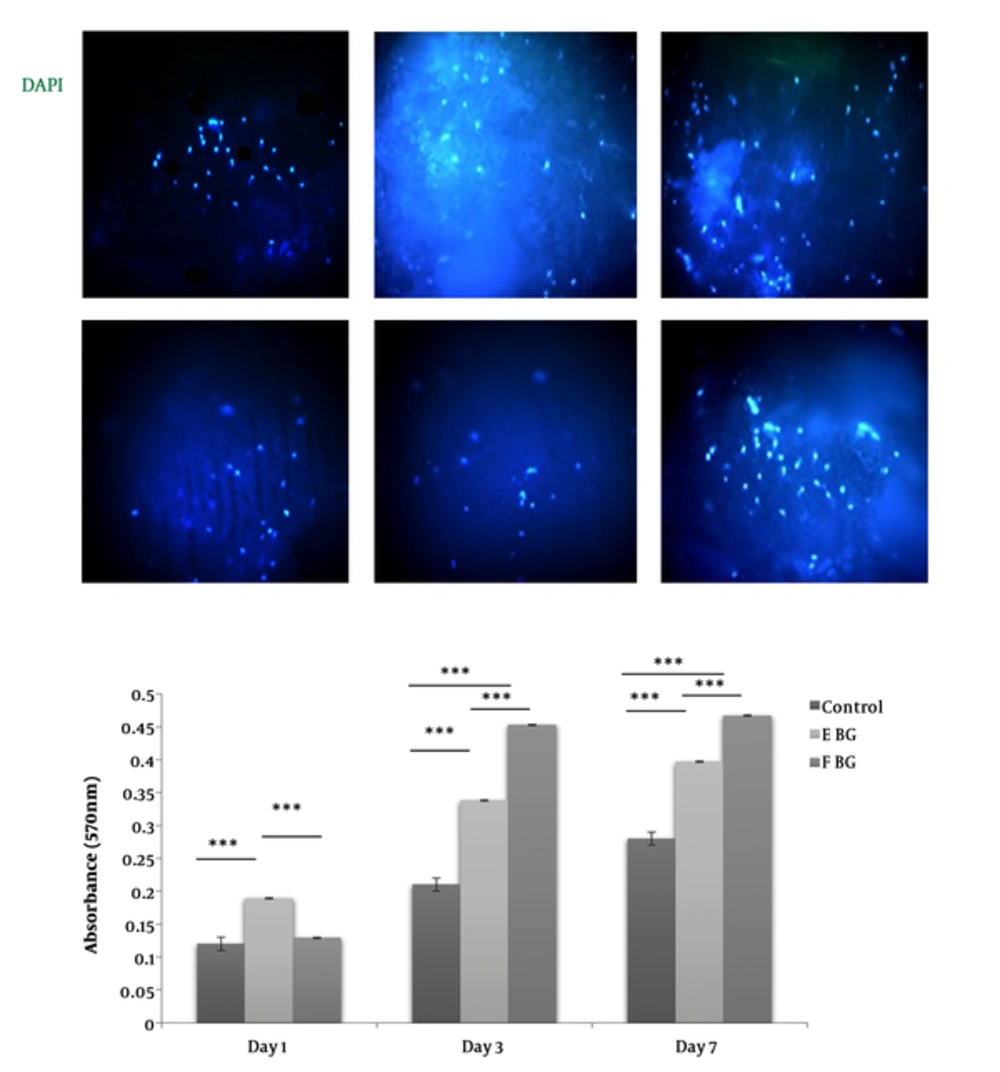
MTT test was employed to determine the viability of cells on days 1, 3, and 7 of being in culture on the scaffolds. As it is shown in Figure 5, both of the scaffolds did induce any negative impact on the proliferation level of the osteoblasts compared to the cells seeded on plastic areas. In addition, the results of the present study indicated that freeze-dried scaffolds were more biocompatible compared to those constructed using the electrospinning method. In general, the results of MTT assay showed that the none of the scaffolds had a toxic effect on the cells and they are both suitable to be used in bone TE (Figure 5B).
proliferation assay for cultured cell on scaffolds. A, In a DAPI test, the cells nuclei are marked in blue color. Relatively more blue spots show more viable cells on the scaffolds surface.Freeze-drying scaffold represents more viable cells and blue spots. The results of DAPI test and MTT test confirm each other. B, The results of MTTassayon 1st, 3rd, and 7th post-seeding days. The statistical analysis has been done at any given days among the samples. Just in first day the cell viability of electrospining scaffold sample was significantly more than the other samples (**P < 0.01).The cell viability for the freeze-drying samples was significantly upper than the other samples on 3rd and 7th post-culturingdays (** P < 0.01, *** P < 0.001).
3.3.4. Cell Viability and Proliferation Test
DAPI staining of the seeded cells on PLGA/Bioglass scaffolds that was done on days 1, 3, and 7 revealed that cells were adhered on both scaffolds (Figure 5A).
3.3.5. Real Time-Polymerase Chain Reaction (qRT-PCR)
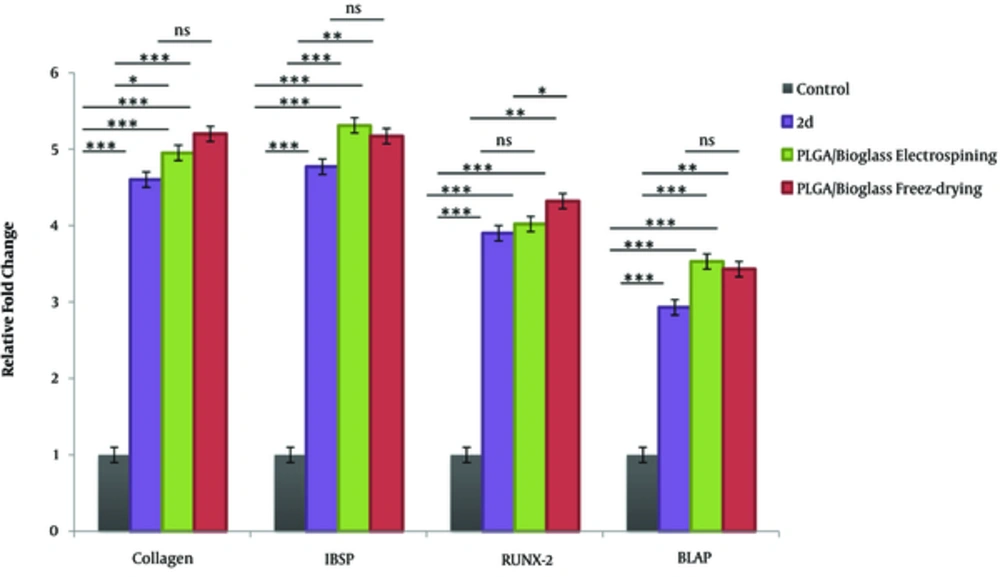
Real-time PCR was carried out to survey the expression levels of osteoblast markers at mRNA levels. The results of RT-PCR showed that osteoblast cells differentiated from endometrial stem cells on PLGA/Bioglass scaffolds expressed phenotypic markers, such as collagen I, RUNX2, IBSP, and BGLAP (Figure 6).
Gene expression analysis of osteoblasts biomarkers in differentiated hEnSCs). Differentiated cells were examined for RNA expression of osteoblasts biomarkers 21days after Induction on TCP and 7 days post induction on two different scaffolds. Data showed that obtained cells could significantly express Collagen, ibsp, runx-2, bglap. An expression of these markers is higher in cells cultured on freeze-dried scaffold compare with electrispining and TCP. GAPDH was the housekeeping control gene. Error bars show SD, n = 3 samples (***P < 0.001; **P < 0.01,*P < 0.05).
4. Discussion
Finding an easy and affordable approach to treat bone losses in neurodegenerative disease with no complications, including laborious surgeries as well as transmission of bacterial and viral diseases, is of a great importance. TE is a path to provide such approaches. The aims of this study were to differentiate endometrial stem cells to osteoblast and to measure and compare the extent of biocompatibility of PLGA/BG scaffolds prepared applying two different methods, namely electrospinning and freeze-drying as a new approach for bone reconstruction in neurodegenerative disease. The major issue, which should be addressed is the adoption of a suitable source of stem cells. Stem cells may be extracted from different origins, like adipose tissue, bone marrow, muscle, periosteum, and placenta (26-29). Endometrial stem cells are in fact a group of stem cells (adult stem cell), which are extracted from endometrial tissue of the uterus, which present some features similar to mesenchymal stem cells. Rehabilitation of the endometrial layer of the uterus in each menstrual cycle is due to these cells (17, 30). Currently, mesenchymal stem cells are used in most of the studies. However, Meng et al., (2007), showed that endometrial stem cells are a suitable substitute for mesenchymal stem cells to be used in TE and cell therapy (31). They have declared that endometrial stem cells have a high proliferation rate, maintain normal karyotype after 34 consecutive passage, are easily accessible, and more importantly, they are capable of differentiating each of the three cell layers, namely endoderm, mesoderm, and ectoderm (31). Therefore, these cells were used in the present study to be differentiated to osteoblast. Mobarakeh et al., (2012), and Ebrahimi-Barough et al., (2013), reported in separate studies that endometrial stem cells are capable of differentiating to various cell lines, including neuron, fat, osteoblasts, and oligodendrocytes (25, 32). Ai et al., (2013) have shown that when endometrial cells, which were seeded onto nancomposite Gel/HA biomimetic scaffold were placed in skull bone loss in mice, this hard tissue was efficiently reconstructed (33). Therefore, these cells were used in this study to be differentiated to osteoblast. The results of the present study were in agreement with those reported by Azami et al., (2013), which indicated that EnSCs were successfully differentiated to osteoblast cells using osteogenic differentiation medium (34). Therefore, EnSCsare expected to be suitable candidates for repairing bone loss and their application is more advantages compared to the other stem cells. Beside selection of the proper stem cells, choosing suitable biomaterials for making a scaffold is one of the major issues for TE (35). The biomaterials used for this purpose should support adhesion, growth, and proliferation of the cells and should also be biocompatible and biodegradable (36, 37). PLGA is one of the recently developed synthetic polymers and its capability to transmit growth factors and induce expression of osteoblast-specific genes have already been proven. Therefore, it is one of the practical tools in bone TE. Another material used in fabrication of this scaffold is Bioglass, which increases bioactivities, including ossification (38). Pamula et al., (2011), who have conducted a study on PLGA/BG scaffold, have reported that the wettability property of the composite scaffold was similar to that of the PLGA. Furthermore, the destruction rate of this composite scaffold was low and the biological properties were appropriate. Therefore, it is one of the highly practical tools in bone TE (22). The other important issue in this field is to choose a proper procedure for making the scaffold. Scaffolds used in bone TE should provide a suitable physical space to accommodate the cells within them and to induce formation of new tissues by exchanging biomolecules (39). Scaffolds should also have a 3D and porous structure with mechanical stability (40). One of the important points in the design of scaffolds is their similarity to extracellular matrix, with respect to morphology and structure (41). As it has been shown in SEMimages, electrospun scaffolds with nanofiber structures are more similar to extracellular matrix and they are expected to provide more appropriate conditions for cell growth. However, the results of MTT assay have revealed that the scaffold prepared using freeze-drying method present a more suitable biocompatibility due to its high porosity, which results in providing suitable conditions for cell adhesion. Moreover, qRT-PCR results have shown that the expression level of RUNX-2 gene, which is one of the main osteoblast-specific genes and was significantly different in two scaffolds.. This indicates the higher advantage of the scaffold prepared using freeze-drying procedure. Lu et al., (2013), stated that most of the extracellular proteins, such as collagen, have a fiber structure with nanosize in in vivo conditions (50 - 500 nm) in diameters, which increases cell adhesion, proliferation and differentiation (42). Nanofiber biomimetic scaffolds have biodegradable polymer nanofibers, which are made via various methods, such as electrospinning, phase-separation and self-assembly, which can imitate the nanofiber structure of the extracellular matrix (42). Furthermore, Lu et al., (2013), stated that the freeze-drying method has been widely used within the recent two decades to make 3D porous scaffolds to be used in TE (42). The advantages of this method include using water and ice crystals in construction of the scaffold instead of using organic solvents (42). As it is mentioned above, this study aims at differentiating EnSCsto osteoblast followed by comparing the two methods used to construct the scaffolds. The results obtained from alkaline phosphatase, alizarin red, and ICC tests, which are shown in Figure 3, indicated the successful differentiation of endometrial cells to osteoblast. However, in order to compare the two scaffolds, the results of MTT, SEM, DAPI, and qRT-PCR should be taken into consideration. SEM images have revealed that both scaffolds have appropriate adhesion properties of the cells. The results obtained from qRT-PCR have shown that the respective genes have been expressed in both scaffolds and that the differences between the two scaffolds are limited. However, the results of the MTT test have indicated that the biocompatibility of both scaffolds are suitable. Although, freeze-dried scaffolds has a higher biocompatibility property compared to those constructed via electrospinning, which may be attributed to its porous structure.
4.1. Conclusion
According to the results of the present study, hEnSCs differentiated to osteoblast like-cells using osteogenic media. hEnSCs exhibit several important and potential advantages over other stem cells, therefore, they can be an attractive alternative candidate to repair bone tissue defects. Hence, hEnSCs are newly known stem cell origins for bone TE in vitro, especially when developed on PLGA/Bioglass scaffolds. Thus, usage of hEnSCs for bone restoration is a new therapeutic approach for interim bone loss in neurodegenerative diseases. It can also be concluded that the PLGA/Bioglass scaffold can provide a suitable 3D structure for translocation of osteoblast cells and the Bioglass material in the scaffold can increase the expression of bone-specific genes.