1. Background
One of the neurological disorders, which can cause neural damage or loss, is epilepsy which effects almost 50 million people, worldwide (1, 2). Near one-third of epileptic cases never achieve remission against antiepileptic drug therapy (2, 3). Drug-resistant epilepsy (DRE) involves pharmacological resistant patients with the failure of adequate trials of two tolerable and appropriate chosen antiepileptic drugs, whether as monotherapy or in combination, in order to reach seizure freedom (according to the International League Against Epilepsy) (4). Furthermore, DRE results in disability and diminished quality of life and leads to physical, psychological, and social impairment along with increased drug burden and mortality (5-9). More than half of the cases of DRE in adults are localization-related epilepsies (10). Electroencephalography (EEG) and video EEG are still the main modalities to detect epileptic abnormalities, type of epilepsy, the epileptic focus, and to recognize non-epileptic events (11, 12). In epileptic cases, MRI has sensitivity varied between 80% and 100% in detecting structural lesions (11). To optimize sensitivity of MRI in localization of structural etiologies and definite site for surgery, it should be performed using a proper epilepsy protocol (13, 14). The most common epilepsy-specific MRI protocol includes slice thickness of 3 mm or less of T2Weighted sequence and FLAIR (fluid-attenuated inversion recovery) sequence orientations for hippocampal angulation and T1Weighted sequence (3D volume with 1 mm isotropic voxel size). Recently, susceptibility weighted imaging (SWI) has been considered as a supplementary method for MRI in cases with DRE. This method is a high resolution, 3D-gradient echo MR imaging, which is sensitive to changes in the local magnetic field (15). Lesion composition influences changes of phase images in SWI (16).
Susceptibility weighted imaging (SWI) administers innovative sources of contrast enhancement, visualizing changes in magnetic susceptibility, which are caused by different substances, such as iron, hemorrhage or calcium. The basic opinion of this technique is preserving phase information to the final image, discarding phase artifacts, and keeping only the local phase of interest. Three main superiorities of SWI when compared with T2*-weighted GE technique include (1) long echo time (TE), (2) high-resolution flow-compensated, and (3) three-dimensional (3D) imaging with filtered phase information for each voxel (17). As usual, most conventional MR sequences apply magnitude information for sequencing. Although phase data are produced each time and consist of potentially useful information, they cannot be used for clinical purposes due to the effects of background magnetic fields. The phase images are sensitive to changes in the local magnetic field, i.e. susceptibility, because of blood and its various products along with other substances, such as calcium. By passing the data through a high-pass Hanning filter, it has become possible to remove the unwanted artifacts while retaining the local phase of interest. The remaining valuable phase data is then combined with magnitude images multiplied several times, and eventually produces SWI images (18). Therefore, SWI is especially helpful in differentiating calcified lesion from microhemorrhages, which are both characterized by low signal; calcifications due to a positive phase shift appear as bright lesions, whereas hemorrhagic lesions appear dark due to a negative phase shift (17). Moreover, SWI is an appropriate technique for the detection of vascular lesions, such as telangiectasias, and the caput medusa of venous angiomas as a result of phase changes due to slow flow and deoxyhemoglobin concentration (19).
In this study, the MR protocol was dedicated for epileptic patients. The SWI sequences were also used while these images were not routinely used for epileptic cases. A recent study demonstrated that SWI images may increase the probability of detecting calcified and vascular or hemorrhagic lesions better than conventional MRI (15). As there is little data regarding application of this method along with MRI in DRE cases, the current study was designed to compare the power of SWI with epilepsy protocol conventional MRI in detecting epilepsy inducing lesion in DRE patients.
2. Methods
2.1. Study Design
This retrospective cross sectional study was carried out at the Neurology Ward of Imam Khomeini Hospital (affiliated hospital of Tehran University of Medical Sciences) and Haghighat imaging center (which is a referral center in Tehran for DRE patient) between August, 2014 and March, 2016. The number of patients admitted to Imam Khomeini Hospital was 1254 (22 patients were included in the study) and 2301 in other centers (37 patients were included in the study).
2.2. Inclusion and Exclusion Criteria
Patients with drug-resistant epilepsy (no improvement after application of two antiepileptic drugs with maximum doses for at least six months) were selected for the study. The researchers retrospectively selected patients, who underwent both SWI and MRI for their pre-surgical evaluation. The exclusion criteria were age of less than two years or more than 80 years old and presence of previous intracranial surgery and claustrophobia.
2.3. Magnetic Resonance Imaging Method
Cranial MR imaging was performed with a 1.5-T superconducting system (Avanto, TIM [76X18], Q Engine; Siemens, Erlangen, Germany) by using a 12-channel phased array head coil. T2-BLADE [4000/106 ms (TR/TE) NEX 1] was performed in the axial plane by using a 0.5-intersection gap, and 5-mm section thickness. In addition, T2-weighted [4,180/94 (TR/TE), NEX 1] images in the sagittal plane, T2-weighted [4,110/91 (TR/TE), NEX 1, slice thickness 4 mm,] images in the coronal plane, Isotropic T1 3D MPRAGE [2300/326(TR/TE), NEX 1, voxel size 1 × 1 × 1 mm] images in the axial plane, and Isotropic 3D FLAIR [5000/360/1800 (TR/TE/TI) NEX 1 ,voxel size 1 × 1 × 1 mm ] images in the sagittal plane were acquired. SWI 3D [49/40 (TR/TE), NEX 1, slice thickness 2 mm] was performed in the transverse plane to look for any susceptibility effects resulting from the presence of calcium or hemosiderin in the lesion. Two experienced neuroradiologists, who were blinded to the EEG and medical history reviewed all the images. Data regarding age, gender, EEG, type of seizure, age at seizure onset, and seizure frequency during a month were recorded for all cases. Data analysis was performed by means of SPSS version 22 (Statistical Package for the Social Sciences, version 22, SPSS Inc, Chicago, Illinois, USA). Signal characteristics and analysis of paramagnetic and diamagnetic substances on phase images were evaluated by reviewers, as described by Deistung et al. (20).
2.4. Inter-Observer Agreement
Inter-observer agreement among primary reviewers was determined using Cohen’s kappa scores. Agreement was scored as follows: agreement (< 0), slight (0 to 0.20), fair (0.21 to 0.40), moderate (0.41 to 0.60), substantial (0.61 to 0.80), and almost perfect agreement (0.81 to 1).
2.5. Ethical Issues
Selected patients were asked to fill informed consent forms. The study had been approved by the ethics committee of TUMS.
2.6. Statistical Analysis
Descriptive values are presented in three tables. McNemar χ2 test was used to compare the proportion of patients localized by conventional MRI versus SWI. The SPSS statistical software package (version 18.0) was used for all the data analysis.
3. Results
3.1. Demographic Information
During the study period, 59 cases met the criteria. Thirty-four were male (57.6%) and 25 were female. Basic characteristics are summarized in Table 1.
| Characteristics | All Implanted Patients (N = 59) |
|---|---|
| Age, y | 30.1 ± 15.3 |
| Number of males/females | 34/25 |
| Age at first seizure, y | 11.5 ± 15.8 |
| Duration of epilepsy, y | 18.5 ± 9.7 |
| Number of AEDs at enrollment | 3.4 per patient |
| Mean seizure frequency (seizures/month) | 21.2 ± 40 |
| Seizure type (number of patients), % | |
| Simple focal | 15.25 |
| Simple and complex focal | 38.98 |
| Simple, complex, and secondary generalized | 32.20 |
| Complex focal | 13.56 |
Abbreviations: AEDs, antiepileptic drugs; SD, standard deviation.
a Values are expressed as mean ± SD.
Inter-Observer Agreement
The inter-observor agreement (kappa score, Fleiss kappa) for chosen region was κ = 0.675 (95% CI, 0.441 - 0.949), P < 0.001 vs. κ = 0.719 (95% CI, 0.501 - 0.937), P < 0.001 (SWI vs. MRI, P value < 0.05).
In 50 cases (85%), there was evidence of brain abnormalities in conventional MRI and/or SWI. Brain abnormalities were evident in conventional MRI evaluation of 47 (79%) cases. Three out of twelve cases that had normal MRI had brain abnormalities in SWI (Figures 1 and 2). Same lateralization or localized lesions were detected in EEG and MRI found in 20 (40%) cases. In 13 cases, SWI added more information to conventional MRI findings.
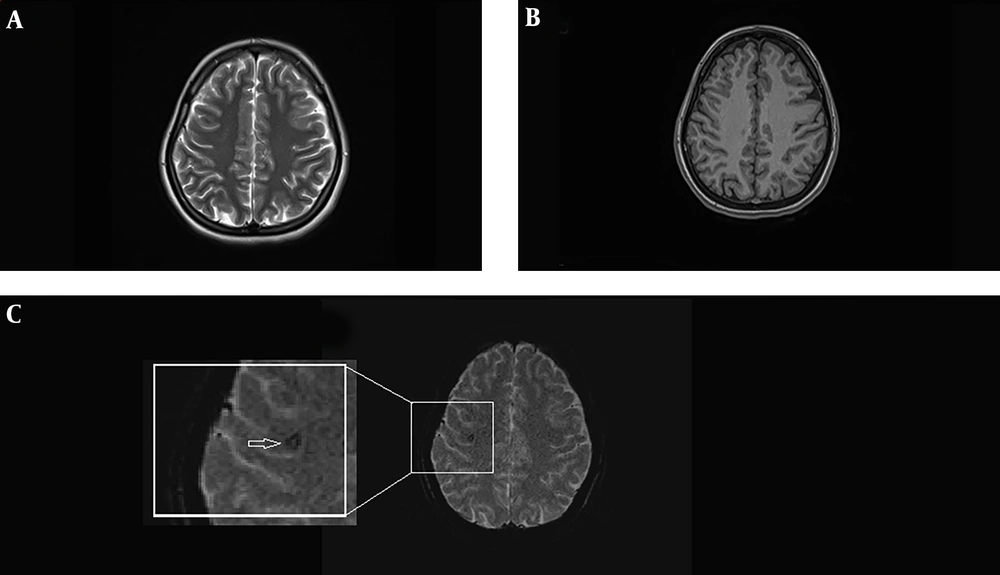
MRI of a 25 years old female with history of complex partial seizures from 16 years ago whose EEG study showed epileptiform discharges originating from right temprofrontal region. Axial T2-weighted (A) and axial T1-weighted MRI (B) are normal. In the SWI sequence (C) focal signal abnormality is detected in the right frontal lobe subcortical white matter (arrow). It has signal void configuration and probably compatible with tiny cavernoma.
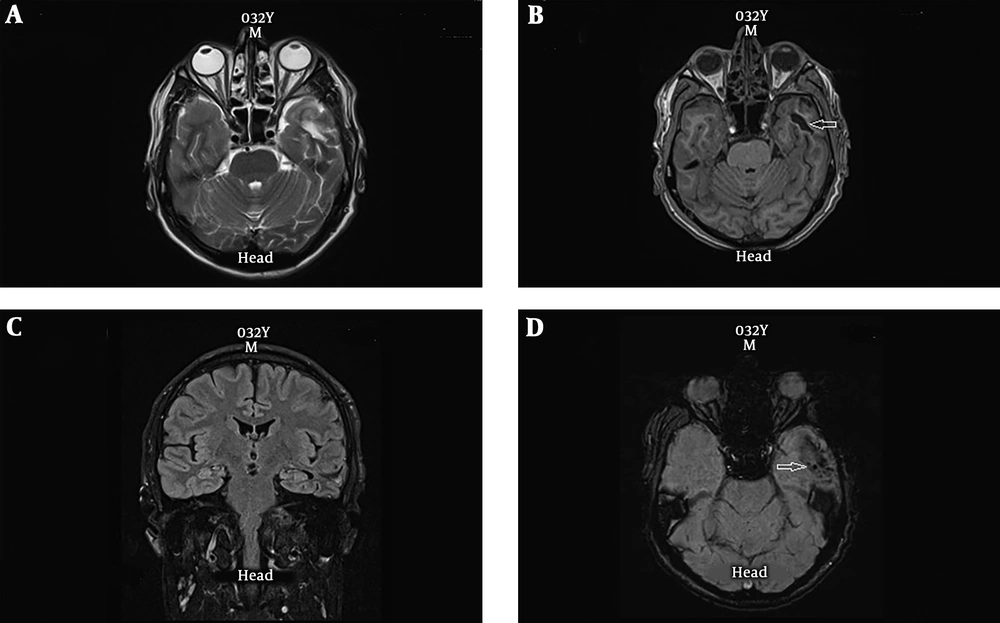
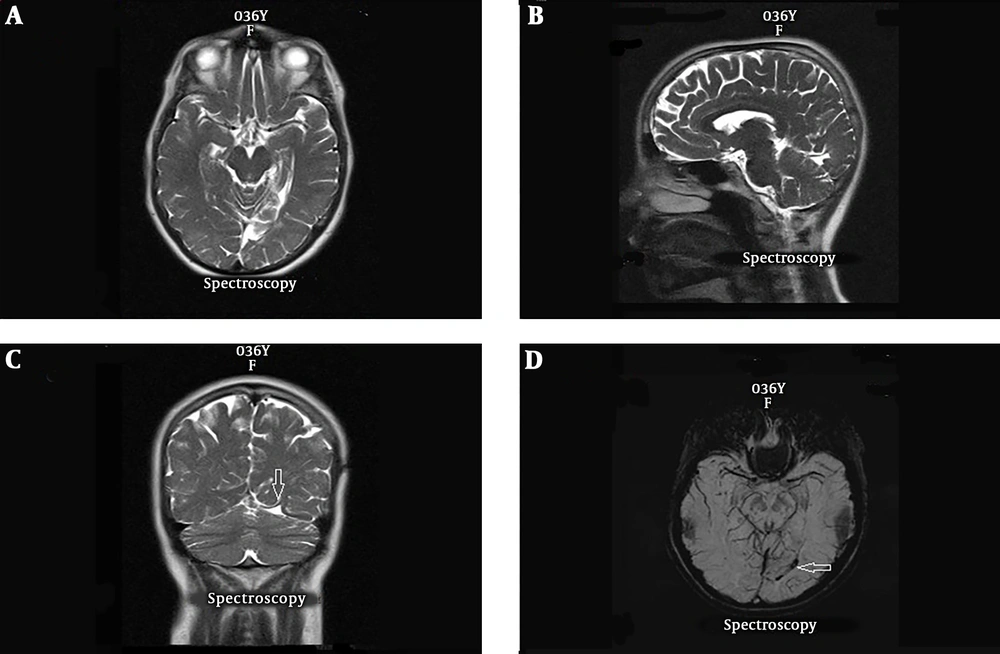
A 39 years old female with history of generalized seizures from 28 years ago with evidence of seizure originating from right parietal area in EEG study. Axial T2-weighted (A) and axial T1-weighted MRI (B) are normal. A cluster of abnormal vessels are present in the right occipital lobe parasagital aspect (arrow) which are only detectable in the SWI sequence (C).
In two cases, EEG showed localized lesions and partial seizures, and conventional MRI showed no abnormality while SWI showed abnormal vascular cluster (Figure 2). In one of these two cases, caput medusa in agreement with developmental venous anomaly, was reported. In another patient, conventional MRI was normal while SWI showed evidence of cavernoma (Figure 1). One of the cases, in addition to the lesion detectable in conventional MRI, SWI showed two extra signal void lesions in agreement with cavernomas.
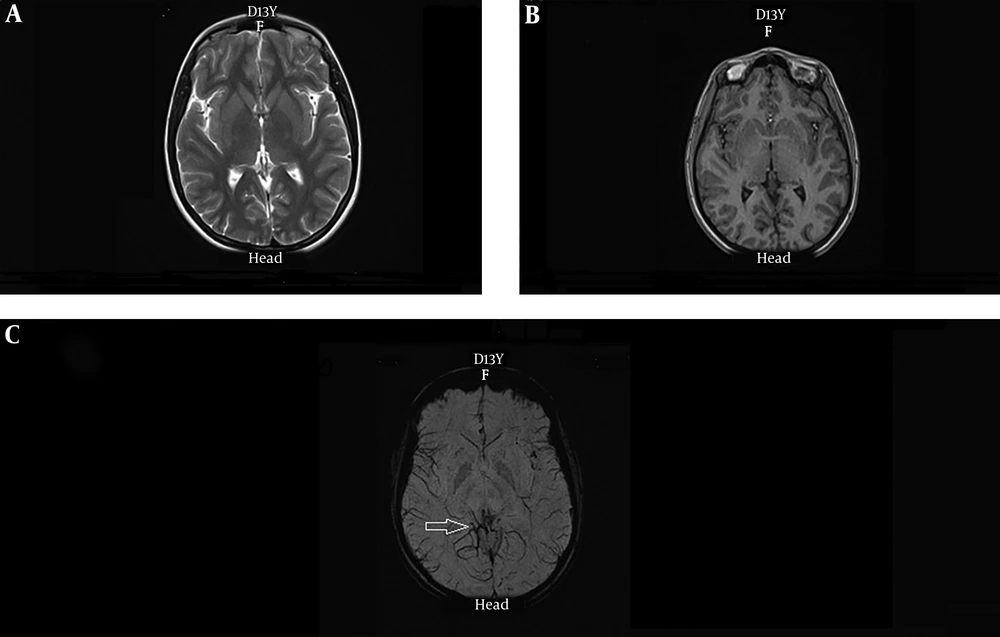
In four patients, conventional MRI showed hyper-signal lesion and, SWI also showed evidence of AVM or cavernoma (Figure 3). In one patient, evidence of volume loss was seen at the left temporooccipital lobe and SWI detected hemosiderin deposition in this region and probability of previous hemorrhage was proposed while MRI only showed evidence of gliosis and signal abnormality (Figure 4). Conventional MRI showed brain mass in the right temporo-parietal region, in which SWI reported evidence of bleeding in one of the other cases.
MRI of 20 years old male with history of generalized seizures from the age of 11 and frequency of 7 seizures per month. Axial T2-weighted (A) and axial T1-weighted MRI (B) showing focal signal abnormality in the left temporal lobe peripheral aspect (arrow), associated with gliotic component in coronal T2-weighted fluid-attenuated inversion recovery (FLAIR) (C). In the SWI sequence (D) accompanying hemosiderin deposition and hemorrhagic product is seen (arrow). There is also prominence of cortical vessels which may suggest associated underlying AVM or post traumatic ICH.
A 34 years old female with history of 5 complex partial seizures per month from the age of ten and evidence of partial seizure originating from left temporal region. Axial, sagittal and coronal T2-weighted images (A-C) showing signal abnormality and volume loss in the left temporooccipital lobe which is associated with gliotic component (arrow). SWI sequence (D) represent associated hemosiderin deposition in this region (arrow) probably related to previous hemorrhagic event.
A 22-year-old male with history of complex partial seizures for 15 years had two lesions in conventional MRI. One of these lesions was in the right ventricular sub-ependymal layer and based on its signal pattern, calcified nodule was suggested; however, in SWI, the lesion had hypo-signal components in favor of focal hemosiderin depositions. In two cases, one old lesion with gliotic border was reported in conventional MRI, whose SWI showed slight signal void abnormality in the lesion in favor of remote traumatic lesion and sequel of previous intra cranial hemorrhage (ICH), respectively.
3.3. McNemar’s χ2 Test
The McNemar’s test indicated that there was a statistically significant difference in localization and/or type of lesion in seizure foci when using MRI only versus using MRI and SWI together; the P value was < 0.01 (χ2 = 26.21). Also, the lesions were divided to two categories, including temporal lobe lesions and non-temporal lobe lesions. McNemar’s test showed a significant difference between MRI and SWI (each methods alone) when lesions were located in temporal lobe (P value ≤ 0.001), in contrast there was no significant difference between the two methods for lesions localized in other parts of the brain (P value = 0.146).
4. Discussion
In this study, more information from SWI was reported in 13 (22%) patients. Three out of 12 cases that had normal MRI (in conventional MRI with epilepsy protocol), had brain abnormalities in SWI (Figures 1 and 2), and in 10 out of 47 patients, who had brain abnormality in MRI, SWI helped to better identify the underling lesion, such as vascular anomaly, as the most common pathological finding, and/or brain traumatic injury (BTI). In one of the patients, conventional MRI was normal while SWI showed evidence of cavernoma (Figure 1). In another case, in addition to the lesion, which was detected in conventional MRI, SWI showed two extra signal void lesions, which were in agreement with cavernomas. In two patients, SWI detected hemosiderin deposition in this region and probability of previous hemorrhage (traumatic injury) was proposed while MRI only showed evidence of gliosis and/or signal abnormality. Also, statistical analysis by McNemar’s test illustrated that there was a significant difference in localization of seizure foci when using MRI only versus using MRI and SWI together; especially for the lesion localized in the temporal lobe.
In this study, adding SWI to the MRI epilepsy protocol study resulted in more information in 22% of the cases. Three out of 12 DRE cases with normal MRI had brain structural abnormalities in SWI. Also, in 10 out of 47 patients, SWI helped better identification of the underling lesion. Importantly, the findings strongly confirm earlier studies. Saini et al. evaluated the addition of T2*gradient echo/susceptibility weighted imaging (T2*GRE/SWI) sequence to a dedicated MRI protocol. In 16 out of 137 patients, the T2*GRE/SWI sequence gave additional information. In that study, the only remarkable finding was focal calcification and only in two cases, SWI helped detect vascular anomaly. However, in the current study, vascular anomaly was the main pathological finding in DRE patients by adding SWI to the protocol, which provided additional helpful information (15).
Among localization-related DRE cases, better response to surgical treatment was reported in vascular lesions rather than other lesions, such as mesial temporal sclerosis (MTS), cortical dysgenesis, or dual pathology (21-24). In the absence of prominent hemorrhagic or focal neurological deficit, Cavernous malformations had a higher five-year risk of development of epilepsy rather than arteriovenous malformations, and they usually appeared as multiple lesions in epileptic cases (25). Cavernous malformations are distinguished by reticulated mixed-signal-intensity mass due to presence of hemorrhagic lesions at different ages with a complete rim of hemosiderin. The GRE and SWI techniques are specified for detecting such lesions. In the current study, SWI detected three covernomas, which were undetectable in conventional MRI with routine epilepsy protocol. In another case, focal signal abnormality with gliosis was reported, according to conventional MRI. However, SWI suggested possibility of underlying cavernoma with associated ICH in this region, so provided insight to the real nature of the lesion.
In two of the cases with a normal conventional MRI, evidence of a cluster of abnormal vessels was detected in SWI. Signal void veins with configuration of “caput medusa”, typical of developmental venous anomaly, was reported in one of them. Furthermore, DVA is a variation of normal venous drainage and does not have any proliferative potential or any direct arteriovenous shunt (26). The diagnosis of DVAs is seldom possible on imaging studies, such CT scan and sometimes difficult to be detected in MRI without using contrast (27). As mentioned previously, SWI sequences are appropriate by detecting the phase differences of tiny vascular lesions, such as the caput medusae and telangiectasias, due to combination of slow flow and concentration of deoxyhaemoglobin in sequences (28). Recently, DVA lesions have been suggested as the main localized pathology of focal seizures in DRE cases when no epileptogenic foci can be discovered (29). Probable mechanisms are subclinical hemorrhage accompanied by a cavernous malformation and/or restricted outflow or increased inflow, causing intermittent cortical hyperemia and dysfunction as an epileptic foci (26).
In this study, traumatic brain injury (TBI) was the other pathology, for which SWI sequence helped to better identify the nature of the lesion. Post traumatic epilepsy (PTE) is a common morbidity of TBI, presented as spontaneous and recurrent attacks in a patient following head injury (30). Previous studies demonstrated that significant proportion of TBI patients will develop DRE (31). In TBI patients, SWI has been widely used in the identification of small hemorrhages and improvement of the prediction of outcome (32). Karen et al., who evaluated diffuse axonal injury in children, demonstrated that SWI is a much more sensitive and accurate method rather than conventional T2*-weighted GE sequences in discovering hemorrhagic diffuse axonal injury and may provide better information about the prognosis and duration of coma and long-term neurological outcomes (33). Especially in developing countries, application of such a complementary method to MR protocols will help consider infective and post-traumatic calcified or non-calcified lesions, which could be responsible for DRE patients (15) and, decrease post-operative disability due to the resection of non-lesional functional tissue, resulting in cognitive and memory dysfunction (34). Postsurgical prognoses are significantly different between patients with unifocal and multifocal epilepsy. Seizure-free rate in patients with multifocal epilepsy is low due to incomplete resection of all the epileptogenic zones. Due to the ability of SWI sequence in detecting further epileptogenic areas additional to lesion detectable with conventional MRI with routine epilepsy protocol, as shown in the literature, application of SWI in patients with refractory epilepsy will guide physicians for better identification of ideal candidates for epilepsy surgery and improving postsurgical prognoses.
4.1. Conclusions
In conclusion, SWI, a combination of GE techniques with phase information, is a profitable and helpful technique for the characterization and identification of vascular malformations, traumatic brain injuries and calcifications of infections or low-grade tumors, such as oligodendroglioma. In the current study, cerebral vascular malformation was the most common pathology that SWI sequence helped to detect or better characterize. Although SWI has some inherent limitations, it could be helpful in detecting underlying causes of epilepsy in cases with drug-resistant epilepsy. Therefore, it can be included in routine epilepsy protocol of MRI.