1. Background
Tissue engineering aims to create artificial tissue and organs as alternatives for transplanting damaged tissue parts in the body. For this, scaffold materials play a critical role in guiding cell adhesion, proliferation, migration, and tissue formation (1-3). Until now, various composite scaffolds have been utilized from natural or synthetic polymers such as alginate (Alg), pectin, gelatin, hyaluronic acid, carboxymethyl cellulose, as well as polyvinyl alcohol (PVA), poly ethylene glycol, and poly lactic acid in multiple field (4, 5). In addition, bioactive inorganic materials have guided seeded cells into aim specific cells and tissues. Among these substrates, sodium alginate, a polyelectrolyte chain with negative charges on its backbone, has been extensively used in biomedical, biopharmaceutical, and biofabrication applications, due to its relatively low cost, biocompatibility, and mild gelation behavior with divalent cations (6-9). In addition, PVA is an inexpensive water-soluble polymer that has excellent physical properties and chemical stability. Main characteristics of PVA is its high biocompatibility and durability which is attractive in the biomedical field. PVA has been widely used as a useful material for tissue engineering and drug delivery in different shapes and architectures such as fiber, spheroid ranging from tens to hundreds of nanometers. Electrospun ultrafine fibers obtained from a viscous aqueous solution of composite alginate and PVA under mild conditions can be used in cell differentiation and proliferation studies (4-6). In addition, hydroxyapatite (HA, Ca10 (PO4)6(OH)2) is the main inorganic substrate through bone tissue. Nano-structured HA are extensively utilized in musculoskeletal tissue regeneration and repair due to its excellent biocompatibility and bone integration ability (7-9).
Periodontal ligament stem cells (PDLCs), adult stem cells have potential to utilize in regenerative medicine applications, owing to their distinctive localization and differentiation properties into fibrogenic and osteogenic (10, 11). PDLCs have the capacity to differentiate into keratocytes, cementoblasts, and osteoblasts cells in the presence of cell inducing factors and scaffolds. For example, PDLCs shared many osteoblast-like properties, including expression of the alkaline phosphatase as abone-associated marker, response to bone-inductive factors, and the capacity to form mineralized nodules in in vitro. PDLCs behavior in alginate microparticle was provided as a reliable approach for bone tissue engineering and regenerative medicine (8, 10, 11). PDLCs in Chitosan-nanohydroxyapatite scaffold could induce osteogenic and adipogenic differentiation in vitro and in vivo (9). PDLCs cells demonstrated that the porous collagen-HA, as well as gelatin-HA composites, have good biocompatibility and biomimetic properties for bone tissue regeneration. As a result of the factors mentioned above, our hypothesis is that osteogenic differentiation of the PDLCs could promote through composite scaffold, which have a desired feature as seed cells for periodontal bone regeneration in treatment of periodontal disease (8, 10-12).
This study aimed to evaluate the effect of the nanofibrous scaffolds on periodontal ligament stem cells (PDLSCs) differentiation into ostoblast cells lineage. Electrospun fibrous membranes composed of alginate/poly (vinyl alcohol) (PVA) of approximately 300 - 550 nm in diameter, and immobilizing hydroxyapatite nanoparticle (HANPs) were developed; their feasibility, as a functional substrate of osteoblast induction was studied. PDLSCs were induced to differentiate into ostoblast-like cells in the presence of dexamethasone, ascorbic acid, and glycerol phosphate on composite scaffolds. Then, differentiated ostoblast-like cells were investigated morphologically and expression of ostoblast markers was studied using real-time PCR and immunocytochemical analysis for PVA-based hydrogel, Alg/PVA, and Alg/PVA/HANPs based composite hydrogels.
2. Methods
2.1. Fabrication of HANPs and Characterization
HANPs were synthesized based on a method described elsewhere (9). Briefly, calcium hydroxide 7.5% (w/v) was dissolved in a 100 mL volume of 50% (v/v) ethanol at 35°C, whereas solution mixed vigorously using magnetic stirrer for 4 hours. Then, ammonium dihydrogen phosphate 6.5% (w/v) was dissolved in 100 mL distilled water, followed by the addition of the calcium hydroxide solution. The aqueous was precipitated at pH = 11 using sodium hydroxide 1 N. The resultant polymers were then freeze-dried.
2.2. Hybrid Alg/PVA/HANPs Electrospinning Process
PVA were dissolved in Krebs Ringer Hepes-buffered saline (KRH, pH 7.4) at 12% (w/v) and 60°C. To this solution, HANPs and alginate were added at 1% (w/v) and 1% (w/v), respectively, and then stirred for 20 hours at 40°C. After obtaining homogeneous polymer, the solution was made to flow through the 18-gauge stainless steel needles at 0.4 mL/h using syringe pump. The tip of the needle 21-gauge was cut at 90° in an axial direction. The needle was connected to the cathode of a high-voltage DC generator at 20 kV and positioned above a rectangular (20 × 10 cm) aluminum foil, which was designed as a static collector and connected to the ground wire. The fixed distance between the tip of the needle and the surface of the hydrogelation medium was 15 cm during the electrospining process. Acquired nanofibrous mats were crosslinked by calcium chloride and glutaraldehyde at 3% (w/v) and 0.03% (w/v), respectively.
2.3. Degradation Study
The degradability of the fibers over time could provide an estimate of the stability of a scaffold in vitro. For this aim, the nanofiber mats were cut into rectangular stripes and immersed in DMEM containing FBS at 10% (v/v) and distilled water at 37°C until 28 days of incubation. At each time point, the matrices were removed from the media, rinsed several times with deionized water, and freeze dried.
2.4. Mechanical Property
Mechanical properties of obtained electrospun mats including PVA, Alg/PVA, and Alg/PVA/HANPs blended nanofibers were evaluated via tensile test. The electrospun mat in the size of 40 mm gauge length and 15 mm width were cut into dumbbell shaped samples. Then, samples were subjected at a strain rate of 5 mm/min until specimens were ruptured.
2.5. Scaffold Characterization
Morphology and size distribution of nanofibers were investigated using scanning electron microscopy (SEM; Philips XL30, Netherland). The nanofiber specimens were sputter-coated with gold during 40 s at 60 m and current, 25 kV. Diameters of about 40 different locations of scaffolds were measured from randomly selected fibers using the Image J software. Moreover, pore size distribution and percentage porosity of the nanofibers were measured using a mercury intrusion porosimeter. The porosity was calculated according to the relationship between the applied pressure and the pore diameter, into which mercury intrudes further into the sample as well as filling small surface pores. The hydrophilicity of the scaffolds was determined using static contact angle measurements with a sessile drop method by a contact angle measuring system (G10, KRUSS, Hamburg, Germany).
2.6. Periodontal Ligament Stem Cells (PDLSCs) Isolation and Culture
Human PDL cells from human teeth were used to evaluate the cellular behaviors to the synthesized composite scaffold. The human PDL cells were obtained from the explants collected from teeth removed in orthodontic treatment or extracted third molars with informed consent. For this aim, the obtained tooth was washed several times with phosphate buffered saline (PBS), and then the PDL was scratched from the two-thirds of the root surface. The tissues were washed again with sterile PBS, cut into small pieces, and plated into 25 cm2 culture flask and incubated. Cells were sub-cultured when cells are 70% ~ 80% confluent within Dulbecco’s Modified Eagle’s Medium containing 10% fetal bovine serum (FBS) and 100 µg/mL penicillin/streptomycin under standard culture conditions (37°C, 5% CO2, and 95% humidity). The medium was changed every 2 - 3 days.
2.7. Cell Viability and Proliferation Assay
For cell seeding, the rectangular sized nanofibers were placed in a 96-well plate and were then exposed to ultraviolet light (254 nm) for 30 minutes under laminar flow hood for sterilization. PDLSCs cells were seeded with 1 × 104 cells/well onto 96-well plates. The cytotoxicity and the proliferation of the cells on the nanofibers were assessed by 5-Diphenyltetrazolium Bromide (MTT) assay kits (Sigma-Aldrich, St. Louis, USA) respectively. Then PDLSCs were seeded into each scaffold and incubated for indicated time points. The nanofiber mats were softly rinsed with PBS and transferred into new 24-well culture plates. Next, 5 mg/mL MTT reagent was mixed with refresh media and poured into each well for 4 hours. The cultured media was then removed, dimethylsulfoxide was added, and the absorbance value was measured using a multi-well spectrophotometer at 570 nm.
2.8. PDLSCs Differentiation Into Osteoblast Like Cells in 2D and 3D Scaffold
The following study groups were examined, 2D group and 3D group (Alg/PVA/HANPs nanofiber scaffolds). After sterilization of nanofiber scaffolds, the scaffolds were put in the 24 well plates. After cell seeding in the 24 well plates at a concentration of 2 × 104 cells/well, the differentiation media contain DMEM/F12+ FBS 10%, dexamethasone 10 nM, ascorbic acid 50 µg/mL, and β glycerol phosphate 10 mM were added in each well for 21 days. The culture media was changed every 3 days.
2.9. Real Time RT-PCR
The impacts of the PVA, Alg/PVA, and Alg/PVA/HANPs nanofibrous on the expression of four important bone correlated gens were investigated by real-time reverse transcription (RT) polymerase chain reaction (RT-PCR) including bone morphogenetic protein 4 (BMPL), collagen type I (Col1), osteocalcin (OSC), runt-related transcription factor-2 (Runx-2), and alkaline phosphatase (ALP) (8, 11). The cellular constructs were rinsed with saline buffer at predetermined time points one and 21 days after culture, and the total RNA was isolated using Qiazol reagent Kit, according to the manufacturer’s protocol. In following, the cDNA was synthesized by random hexamers using the M-MLV Reverse Transcriptase kit. The resulting cDNA were then each subjected to quantitative RT-PCR and the gene expression was determined using SYBR-Green (TAKARA, USA). The primer sequences used in this study are BMPL, ALP, OSC, RUNX, and COL (I). The primers sequences are shown in Table 1. For Real-time RT-PCR, the SYBR Premix Ex Taq Master mix (Takara) was utilized. Quantification of bone-related gene expression was done in comparison with B-actin as an internal control gene.
| Gene | Sequence | Length, bp |
|---|---|---|
| B-actin | GGGTCAGAAGGATTCCTATG | 20 - 20 |
| GGTCTCAAACATGATCTGGG | ||
| COL (Ι) | F ATGGCTGCACGAGTCACACC | 20 - 20 |
| R CAACGTCGAAGCCGAATTCC | ||
| RUNX2 | F ACTCTACCACCCCGCTGTCTTC | 22 - 21 |
| R AGTTCTGAAGCACCTGCCTGG | ||
| OSC | F GGTGCAGCCTTTGTGTCCAAG | 21 - 23 |
| R AACTCGTCACAGTCCGGATTGAG | ||
| BMPL | FATGGATTCGTGGTGGAAGTG | 21 - 21 |
| RGTGGAGTTCAGATGATCAGC | ||
| ALP | F CCTCGTTGACACCTGGAAG | 19 - 22 |
| R CTGGTAGTTGTTGTGAGCATAG |
2.10. Immunofluorescence Analysis
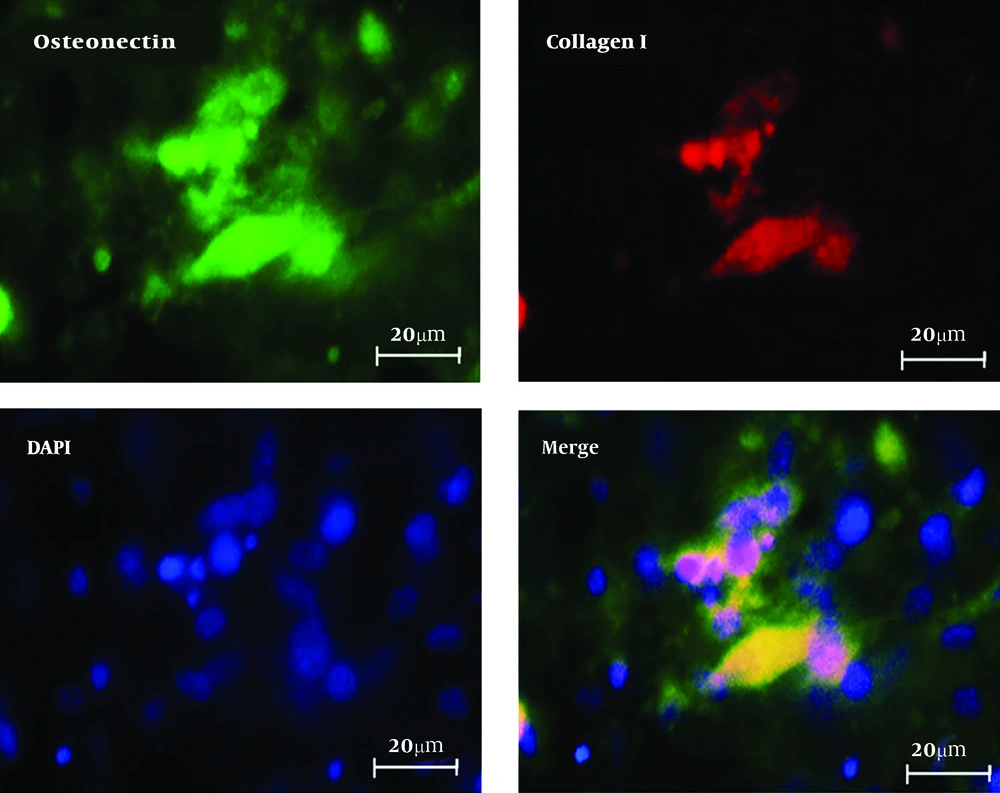
Immunocytochemistry, as duble staining, was used for expression of specific markers for differentiated osteoblast cells. After fixation with 4% PFA (Sigma-Aldrich), cells were permeabilized by 0.2% Triton-X 100 (Sigma-Aldrich) for 30 minutes and then blocked with 5% bovine serum albumin in PBS. After 45 minutes the cells were incubated overnight with primary antibody collagen I, mouse monoclonal AB (Abcam, 1:200) and osteonectin, Rabbit Monoclonal AB (Abcam, 1:200). Secondary antibody (Alexa Fluor@488 donkey anti mouse IgG and Alexa Fluor@594 donkey anti rabbit IgG at a 1:500 dilutions; Abcam) were used at 37°C for 1 hour. The slides were washed with PBS between each step and nuclei staining were performed using 4-, 6-diamidino-2-phenylindole (DAPI, Sigma). The cells were monitored by using fluorescence microscope (Olympus BX51, Japan).
2.11. Statistical Analysis
Quantitative analysis of reverse transcription qRT-PCR was performed by randomization tests using REST 2009 V2.0.13 software, whereas statistically significant differences between groups were indicated. One-way analysis of variance (ANOVA) was used to analyze differences between treatments and control groups, and significant differences were expressed by p value. A difference between groups was considered statistically significant if P > 0.05. The statistical analysis is presented as means ± SD.
3. Results
3.1. SEM Analysis of Scaffolds
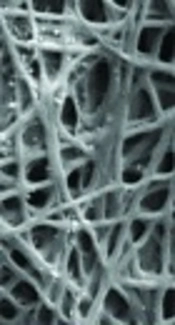
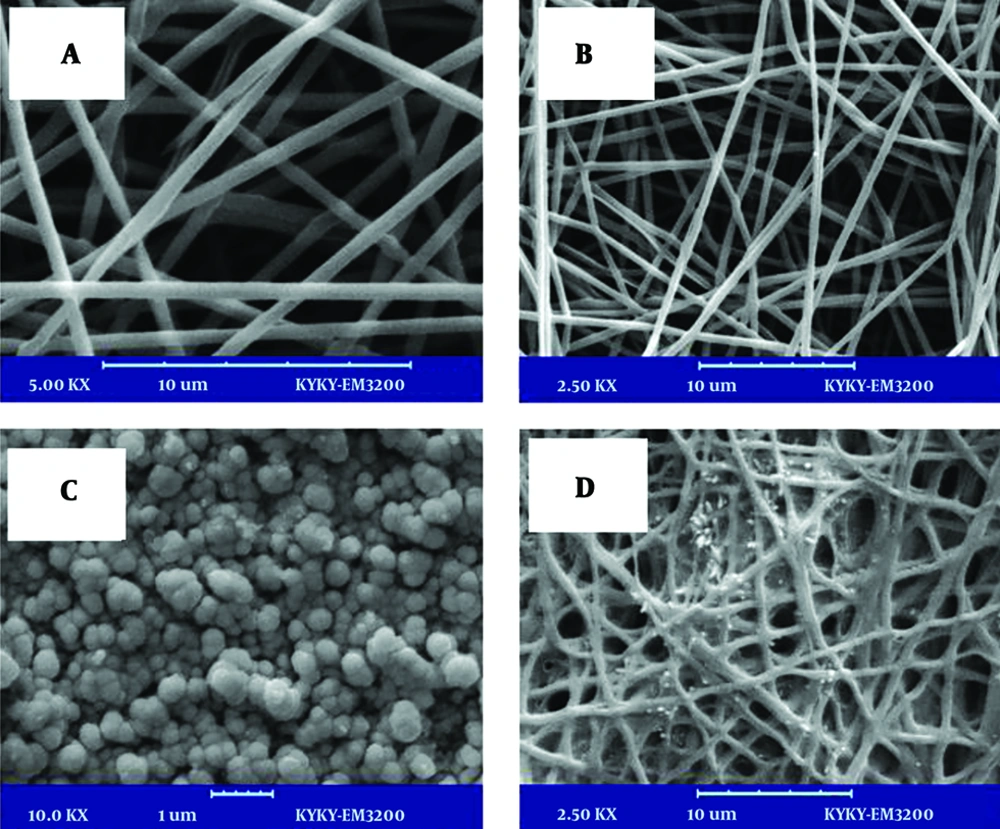
Scaffolds’ microstructure and cell attachment on the scaffolds was evaluated by SEM analysis. The produced fibers in the nano-sized ranged from 300 - 500 nm (Figure 1). The successful electrospinning of PVA co-blending with Alg was achieved and non-woven morphology of is shown in Figure 1. The porosity of nanofiber mats was determined with a simple method by means of image J software and SEM images, which were obtained from different cross sections of the prepared scaffolds. The average porosity of the produced nanofibers was estimated to be 85%. From the results, the addition of 1% (w/v) HANPs to produced nanofibers did not disturb pore structure aperture and porosity of the scaffolds was achieved similar with PVA and Alg/PVA nanofibers. Micrographs captured from the top view of nanocomposites are shown in Figure 1. The high porosity with a high uniform degree of interconnectivity as well as open pore structure was observed in these images. The nanofibers were smooth and beadless in all compositions.
3.2. In vitro Degradation of Nanofibers
Degradation of hydrogel is another important property that determines the stability of the samples under physiological relevant conditions. The nanofibers degraded gradually after incubation in the distilled water and aqueous media until 28 days. Meanwhile, the degradation of pure PVA and Alg/PVA nanofibers were slower than composite Alg/PVA/HANPs nanofibers for 28 days of incubation. The incorporation of HANPs-loaded nanofibers prolonged scaffold degradation. Nearly 30% degradation of Alg/PVA/HANPs scaffolds in distilled water was observed after 28 days and 60% degradation was obtained in case of culture media microenvironment (Table 2). As the porosity of nanofibers was reversed with degradation rate, it is revealed that degradation is highly controlled by surface decomposition of mates. Besides, the contact angle measurement showed that nanofibers containing Alg and HANPs had higher hydrophilic property compared with PVA nanofibers (Figure 2). Therefore, the degradation rate of the polymer matrix was depended on material composition, and porosity (13-15).
| Sample | Media | Weight Loss After 14 Days, % | Weight Loss After 28 Days, % |
|---|---|---|---|
| PVA | D.W | 10.4 | 19.2 |
| PVA | Culture medium | 30.4 | 49.9 |
| Alg/PVA | D.W | 15.5 | 28.4 |
| Alg/PVA | Culture medium | 37 | 56.4 |
| Alg/PVA/HANPs | D.W | 16.9 | 31.3 |
| Alg/PVA/HANPs | Culture medium | 38.1 | 60.9 |
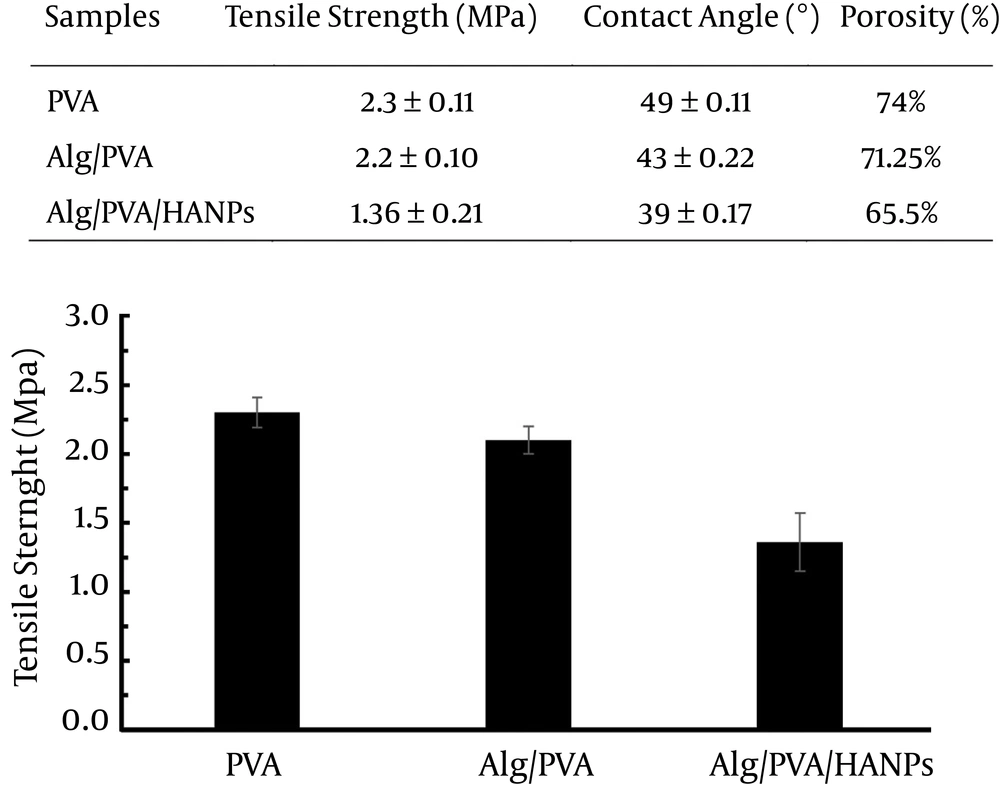
3.3. The Mechanical and Degradation Properties of Nanofibers
The mechanical properties of substrates as the important characteristic regulate cellular behavior including cell differentiation and morphogenesis (13, 16, 17). Therefore, it could be inferred that these nanofibers with different mechanical properties can have different impacts on the cell adhesion, proliferation, as well as migration. The mechanical properties of PVA, Alg/PVA, and Alg/PVA/HANPs nanofibers were calculated through tensile tests and results are shown in Figure 2. Meanwhile composite nanofibers containing HANPs had the lowest level of tensile strength; the PVA and composite Alg/PVA showed 50% and 30% higher stability, respectively. No significant differences between PVA and Alg/PVA samples were observed. The incorporated HANPs increase in stiffness of the mats that consequently leads to tensile strength reduction of scaffold. The results have shown the similar trend for electrospun PCL/HANPs and PLA/HANPs nanofibers (8, 9, 17).
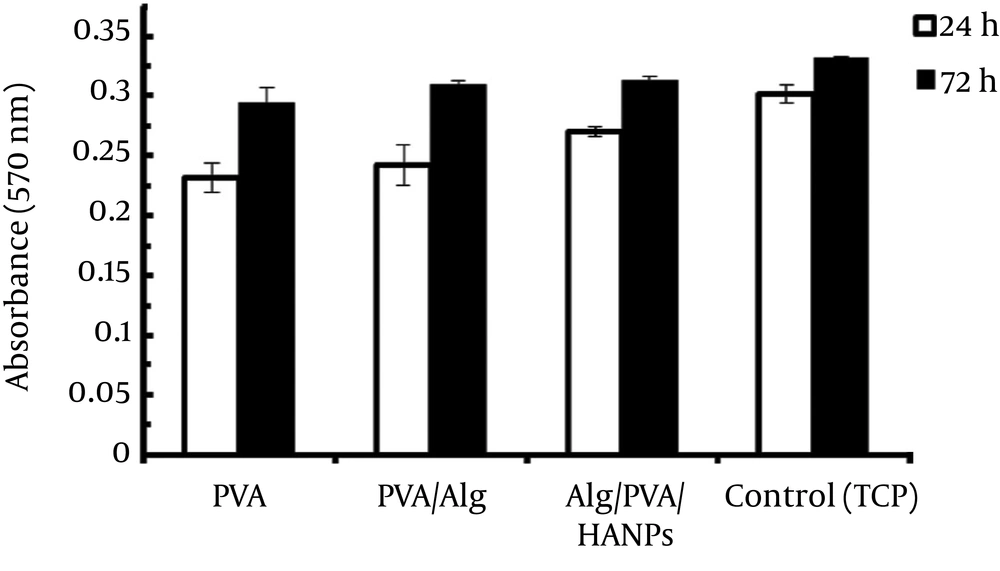
3.4. Proliferation of Cells Cultured on Nanofibers
Proliferation of cells seeded on electrospun Alg/PVA/HANPs composite and pure PVA nanofibers whereas TCP being the control were assessed via MTT assay. As Figure 3 showed, the cell maintained their viability, although the lowest quantity of optical density (OD) was observed for PVA between samples after 24 hours. As the time increased, the difference between the two samples’ behaviors was demonstrated to gradually increase. The OD values for composite Alg/PVA/HANPs sample were significantly higher than those of pure PVA and Alg/PVA nanofibers in 3 days (P < 0.05). This was attributed to the presence of HANPs in the polymer complex structure. The results proved that the addition of HANPs to PVA facilitated cellular growth and positively supported cell proliferation. Besides, OD values for all the nanofiber groups enhanced through extended incubation period, which indicated that all types of nanofibers had good biocompatibility. As matrix stiffness on cell state likely had vital effects for development, differentiation and migration of cells, which were previously reported (15, 18-20). Moreover, recently, the numbers of research have confirmed that HANPs in the polymeric matrix affected cell viability. The HANPs scaffold provided the most favorable substrate for cell growth and composite nanofibers were proper candidates to be used as potential for tissue engineering applications (9, 12, 14). Finally, these results could be verified that cells recognize nanofibers and its microporous structure as well as existence of bioactive HANPs. Therefore, obtained preliminary results in our experiments provided suitable evidence regarding the efficacy of using composites.
3.5. Differentiation of PDLSCs to Osteoblast
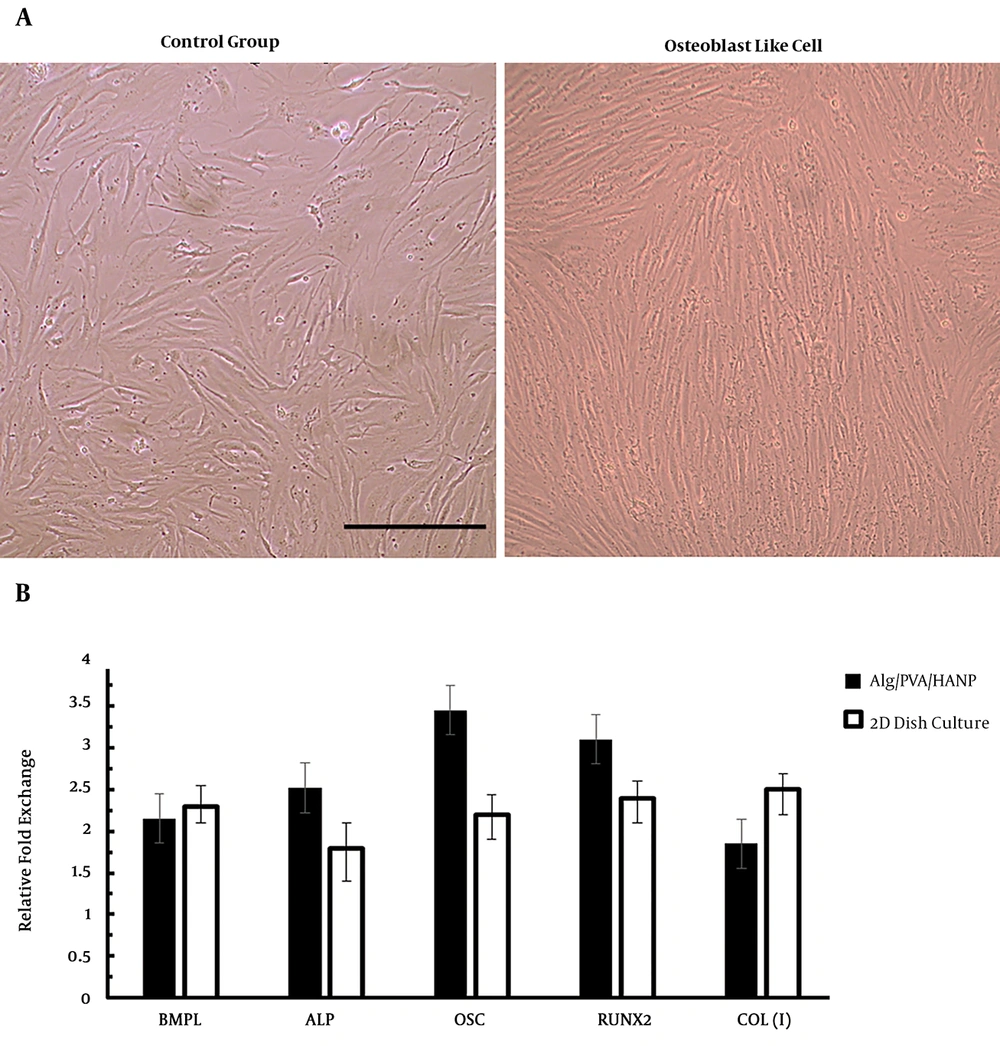
Osteogenic differentiation of PDLSCs was investigated through morphological observation and important osteogenic gene markers measurement by real-time RT-PCR and immunocytochemistry (8, 16). For these tests we used Alg/PVA/HANPs nanofiber scaffold as a better scaffold from other groups because of good features that were shown before. Morphological images showed that the shape of cells changed to long and spindle shape osteoblast like cells morphology (Figure 4A). Highest OSC and Runx2 genes’ expression was observed in the differentiated seeded on Alg/PVA/HANPs during the period of study by real-time RT-PCR (Figure 4B). Immunocytochemistry staining for collagen I and osteonectin for osteoblast like cells derived from PDLSCs on Alg/PVA/HANPs nanofiber scaffolds showed the successful differentiation of cells (Figure 5). This result showed that this scaffold was osteoconductive and can be used for bone defects repair (8, 16, 21).
Osteoblast differentiation of PDLCs into osteoblast like cells. A) morphological observation after 21 days of differentiation in control and differentiated groups (scale bar:100 µm). B) Quantitative mRNA expression analysis of osteoblast-like cells derived from PDLC seeded on Alg/PVA/HANPs nanofiber after 21 days. The expression of osteoblast markers in the differentiated cells onto the electrospun nanofibers was higher than those in the cells cultured onto 2 D culture dish and RUNX2. Results were presented as the mean ± standard deviation (n = 3).
4. Discussion
The purpose of this study was to induce differentiation of PDLSCs into osteoblast using an osteogenic culture media. In addition, various cellular behaviors of differentiated human osteoblast cells on the surface of electrospune Alg/PVA/HANPs nanofiberous were evaluated and compared in terms of cell attachment, morphology, proliferation, as well as gene expression. SEM analysis of electrospun nanofiber revealed uniform morphology in all of the experimental conditions with high degrees of porosity. The evaluation of osteoblast gene expression showed that Alg/PVA/HANPs nanofibers promotes differentiation of PDLSCs into osteoblast cells compared to the PVA, Alg/PVA specimens, and 2D cell culture environments. The results of the present study, in terms of osteoblast-specific gene marker expression including BMPL, ALP, OSC, RUNX2, and COL (I) proved that PDLSCs have the potential to differentiate into osteoblast-like cells. The results of IHC investigation demonstrated that osteocalcin and COL (I) were expressed in the cells treated with osteogenic medium and composite Alg/PVA/HANPs nanofibers. Differentiation of PDLSc into the osteoblast-like cells was confirmed in terms of morphological characteristics and molecular standards. Therefore, integration of HANPs with Alg/PVA nanofibers provided a more suitable scaffold as a biomimetic microenvironment for bone tissue fabrication. The cell viability assays confirmed the ability of PVA, Alg/PVA, as well as Alg/PVA/HANPs nanofibers to support cell viability. The evaluated nanofibers demonstrated proper biocompatibility and cell proliferation rates of osteoblast cells in Alg/PVA/HANPs, which was higher than the control groups.
Moreover, physical features of fiber diameter and pore size of the scaffold are two critical characteristics that play an important role in cellular behavior (19-21). As the size of osteoblasts is about between 10 until 30 µm and cell proliferation comes about in average pore size of 50 µm, whereas cells can infiltrate easily in with pores larger than 100 µm. An average pore size of greater than this size has been recommended to be used due to the enhanced formation and regeneration of bone tissue (12). The fiber diameters of PVA, Alg/PVA, and Alg/PVA/HANPs in the present study were about 300-550 nm. SEM showed that the pristine PVA nanofibers have more porous structures versus the fibrous structure of the Alg/PVA and Alg/PVA/HANPs nanofibers (Figure 1). Meanwhile, the composite nanofibers showed lower pore size and cell infiltration. The PVA nanofibers showed larger pore size, higher porosity, and permeation, which leads to an increase cell proliferation rate (Figure 3). The mechanical property of Alg/PVA/HANPs nanofibers was lower than that of the other nanofibers fabricated in different compositions. The cellular growth and proliferation on the Alg/PVA/HANPs nanofibers provide a more suitable microenvironment for cells during proliferation compared to other nanofibers. It is confirmed that Alg/PVA/HANPs nanofibers are significantly biocompatible and did not exert any cytotoxic effects and also influences the differentiation of PDLSCs into osteoblast-like cells, compared to the other nanofibers. Therefore, Alg/PVA/HANPs nanofibers, as potential bioactive substrates, could be applied for bone tissue engineering applications.
4.1. Conclusion
In this study the PVA/Alg/HANPs composite nanofiber scaffold was fabricated through electrospinning method in order to assess the potential of periodontal ligament stem cells differentiation into osteoblasts. Our findings demonstrated through application of biomimetically synthesized Alg/PVA/HANPs composite could create biologically compatible nanofiber scaffold, which has potential to be used in tissue regeneration and engineering fields.