1. Introduction
Eosinophilic granulomatosis with polyangiitis (EGPA), also named Churg-Strauss syndrome (CSS), is a rare necrotizing systemic vasculitis that affects small to medium vessels (1). This syndrome is also characterized by allergic rhinitis, severe asthma, and eosinophilia (1, 2). American College of Rheumatology defined this syndrome as possession of four of the six following signs: (1) asthma, (2) eosinophilia > 10%, (3) neuropathy, (4) pulmonary infiltrates, (5) paranasal sinus abnormality, and (6) extravascular eosinophil infiltration on tissue biopsy (1, 3). Vasculitis could involve any organ or system; mostly lung (hemoptysis) and skin (rashes, purpura, urticarial), and in lower rates cardiovascular (pericarditis, chest pain), gastrointestinal (diarrhea, vomiting, nausea), renal, and the central nervous (intellectual or motor disturbances) systems (3, 4). The patients also have rapid-onset heart failure. This finding was a feature of poor prognosis in CSS (1). In order to delay this fatal complication, prompt diagnosis and aggressive treatment with immunosuppressive agents are needed (2, 5).
Its incidence is variably reported from 2.4 to 40 cases per one million people (4). It can affect all age groups with the average of 35 to 45 years old. Also, males are more commonly affected than females (4).
The exact etiology of CSS is unknown yet, but immune complexes are probably involved (2). It is believed that CSS could be due to autoimmune reaction to environmental or drug agents (4).
Their anesthetic management could be complicated by cholinesterase deficiency, hypersensitive airway, and multi-organ failure (2). Symptoms such as asthma, pulmonary infiltrates, and allergy could challenge general anesthesia; whereas mono- or polyneuropathies could be contraindications for regional anesthesia (4).
The current study reported the anesthetic management of a patient with CSS undergoing surgery due to schwannoma of T12 with large hepatic hemangioma and special emphasis on its anesthetic management as well as therapeutic protocol. To the authors' best knowledge, it was the first report of a case with a myriad of symptomatology.
2. Case Presentation
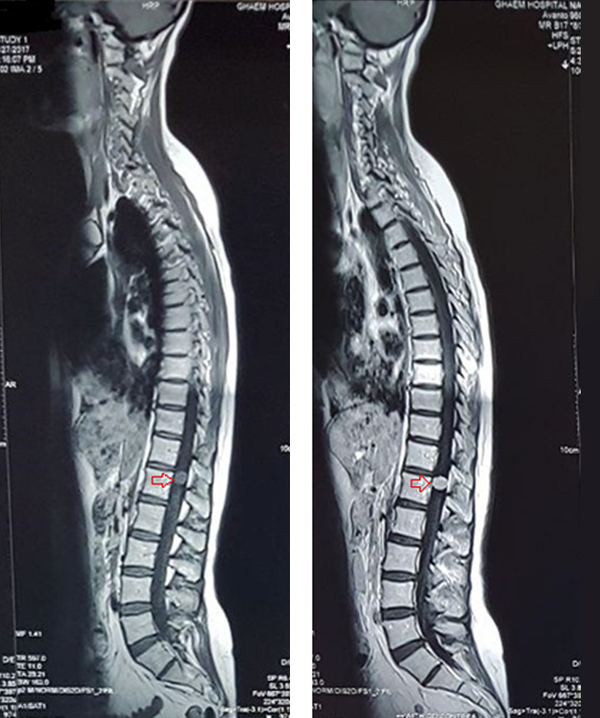
A 42-year-old female with the body mass index (BMI) of 20 kg/m2 weighing 51 kg was admitted for surgery of schwannoma of T12, which caused severe back pain with referral to the lower extremities. She was diagnosed with CSS 20 years ago with complaints of dyspnea, productive cough, and sinusitis. Her diagnosis was confirmed by tissue biopsy of quadriceps and sural nerve. She also had a large hepatic hemangioma in the left lobe since last year, which caused pain in her right upper quadrant (RUQ) and fullness after meal. She had a history of paranasal sinus retention cyst and generalized osteoporosis with nasal bone fracture. She was infertile and had a premature menopause. She took corticosteroids and cyclophosphamide to treat CSS, and alprazolam and asentra for depression and anxiety disorders. On admission, asthma was controlled and on physical examination, paresthesia was observed in both legs. On hematologic examination, leukocytosis (16.200/mm3) and eosinophilia (30%) were detected. Liver and kidney function tests and serum electrolytes were within normal limits. Chest computed tomography (CT) scan and magnetic resonance imaging (MRI) were normal without any patchy infiltrations although schwannoma at T12 was noted (Figure 1). In the transthoracic echocardiography, normal systolic function with mild pericardial effusion was noted. All other investigations were within normal range.
The patient was premedicated with intravenous midazolam 1 mg, fentanyl 150 µg, lidocaine 60 mg, and hydrocortisone 100 mg upon arrived to the operating room. After preoxygenation with 100% O2, anesthesia was induced with etomidate 12 mg and cisatracurium 8 mg. Intubation was successfully performed with endotracheal tube No.7 by aid of direct laryngoscopy. Standard monitoring (electrocardiography (ECG), non-invasive blood pressure, blood oxygen saturation (SpO2), capnography) and invasive blood pressure monitoring via left radial artery were placed. Anesthesia was maintained with O2 100% and infusion of propofol 100 - 150 µg/kg/minute and remifentanil 5 - 25 µg/hour. Afterwards, the patient was positioned prone with special care and suitable roll. Intermittent pneumatic compression (IPC) for deep vein thrombotic (DVT) prophylaxis was employed by the current study.
Throughout the four hours operation, peak airway pressure varied from 15 to 25 cm H2O and hemodynamic variables and arterial blood gas levels were satisfactory. The amount of blood loss during surgery was about two liters replaced by infusion of two units of packed cell plus 1500 mL of 0.9% saline and 1500 mL of Ringer solution.
At the end of surgery, propofol and remifentanil infusions were stopped and the patient was extubated in a deep plane of anesthesia. Oxygen was supplemented by face mask till patient regained full consciousness. Her postoperative period was uneventful in the intensive care unit (ICU).
3. Discussion
CSS is characterized by necrotizing vasculitis of multiple organs, and its prominent symptoms are: asthma, eosinophilia, and extravascular granuloma. The natural history could be divided into three stages: I- late-onset allergic disorders without any personal or familial history with symptoms such as asthma, drug reactions, and allergic rhinitis; II- tissue and blood eosinophilia; III- vasculitis (6).
CSS treatment often involves high dose of corticosteroids plus other immunosuppressive drugs; hence, the response varies in patients (7). Gold standard of treatment is steroids and induction of therapy that could involve cyclophosphamide with maintenance of azathioprine. Other medications involve rituximab, mepolizumab, and omalizumab (7).
In the preoperative period, obtaining pulmonary function test in symptomatic patients is helpful in pulmonary risk assessments. In symptomatic patients, combined treatment with corticosteroids and β2-adrenergic agonists could improve the outcome (8). Cardiac involvement is reported as a major cause of death; therefore, cardiac assessments should be performed in such patients to rule out eosinophilic endomyocarditis, coronary dissection, coronary vasculitis, conduction abnormalities, congestive heart failure, and pericardial effusion.
Anesthesia management should be tailored in order to decrease stimulation of airways and ensure smooth intubation. For these goals, propofol is a good choice to induce anesthesia, as it does not stimulate airway reflexes. Volatile anesthetics except desflurane could be used as maintenance agents due to its bronchodilatory effects. Fentanyl and its derivatives rarely cause histamine release; therefore, they can be used safely in such patients. Neuromuscular blockers with minimal histamine release such as rocuronium, vecuronium, and cisatracurium can be used in patients with CSS. Two cases of cholinesterase deficiency were reported in patients with CSS; therefore, care should be taken when using succinylcholine in such patients (9). Anticholinesterase medication could also increase the risk of secretions, bronchospasm, and airway resistance; therefore, these drugs should be administered with caution.
Chung et al. (8), described anesthesia of a patient with CSS undergoing cholecystectomy. They used Cobra PLA™ instead of endotracheal tube to limit airway irritation. They also used propofol as an induction agent and isoflurane for maintenance due to their bronchodilatory effects. Gurjar et al. (3), also presented a patient with CSS for mastectomy under combined general and thoracic epidural analgesia. They used propofol and isoflurane without neuromuscular blocking agents, and general anesthesia was maintained through the application of laryngeal mask airway. Im et al. (2), reported a case of CSS undergoing endoscopic sinus surgery; they used propofol, sevoflurane, and remifentanil for induction and continued sevoflurane and remifentanil as maintenance agents. They did not use any muscle relaxant; therefore, no anticholinesterase was administered. They only reported a period of hypotension following the induction.
In conclusion, CSS is a multi-organ disorder; the current study believed that tailoring anesthetic plan in order to decrease airway stimulation and also managing chronic corticosteroid usage complication are more useful for patients with CSS (Table 1).
| Comorbidities | Anesthetic Considerations |
|---|---|
| Asthma | Preoperative treatment with corticosteroids plus β-agonist |
| Osteoporosis | Special care for positioning |
| Chronic and high-dose corticosteroids consumption | (1) Vulnerability to infection: care for aseptic techniques, (2) Depressed ACTH: stress dose of corticosteroids |
| Hyper-reactive airway | Anesthesia care: do not induce airway stimulation |
| Pseudo-cholinesterase deficiency | Succinylcholine usage with caution |
| Cardiac comorbidity | Pericardial effusion, LV failure, cardiomyopathies: preoperative cardiac consult to rule out these comorbidities and optimize the patient |
Anesthetic Considerations for Churg-Strauss Syndrome