1. Background
The main purpose of biomaterials fabrication for use in tissue engineering is the simulation of the human body mechanism to revive damaged or diseased tissues. Hard tissues such as bone and cartilages are often in need of reconstruction more commonly because of tumor removal and age-related diseases (1). Cartilage as a hard tissue is difficult to repair because it has no vessel or nerve (2).
Alginate has been extensively used in recent years, especially for cartilage and bone regeneration, because of chemical similarity to the extracellular matrix (ECM) (3-5). The hydrophilic nature of alginate is a significant property that improves its cell compatibility and viability. Furthermore, alginate is a natural polymer that shows better biocompatibility than other synthesized polymers. Sodium alginate scaffolds cross-linked by Ca2+ are widely used for drug delivery and tissue engineering of cartilage because cross-linkers containing calcium ions are safe and do not damage cells (6).
However, the main problem with alginate scaffolds is the lack of appropriate mechanical strength that cannot meet the cartilage tissue requirements (7). The mechanical properties of scaffolds are highly influenced by the interconnectivity of porosities. Furthermore, the controllable degradation rate of fabricated scaffolds at which cartilage tissue regeneration occurs is a key parameter that needs optimization. It has been reported that different physical and chemical properties of scaffolds can affect cell differentiation and osteogenesis ability (8). Freeze-drying is a simple and fast method for the fabrication of different types of scaffolds (9-11). This method can facilitate the optimization of mechanical strength, degradation rate, and osteogenesis potential of scaffolds.
2. Objectives
In this study, we developed a 3D alginate scaffold by the freeze-drying method. To the best of our knowledge, there is no report on the effect of pores architecture produced by freeze-drying method on mechanical properties and cell behavior of alginate scaffolds. Herein, the porosity, degradation rate, and mechanical properties of the fabricated scaffold were optimized in order to use as cartilage substitute. Furthermore, the biocompatibility, periodontal ligament stem cells (PDLSCs) differentiation ability, and cell viability properties of the optimized scaffold were evaluated.
3. Methods
3.1. Alginate Scaffold Preparation
Medium viscosity bio-chemical grade sodium alginate (molecular weight = 30,000, Sigma Aldrich, USA) was dissolved in distilled water to produce different concentrations (4, 8, and 16% (w/v)) under magnet stirring at room temperature for 24 h. Then, 3 mL of 3% (w/v) aqueous calcium chloride was added to the solution as a crosslinking agent, followed by constant stirring at 40ºC for 2 h. The mixture was frozen at -20ºC for 24 h and then freeze-dried (Telstar, Spain) for 48 h.
3.2. Interconnected Porosity of the Fabricated Scaffold
The apparent interconnected porosity of the scaffold fabricated by the freeze-drying method was measured by water displacement principle using Archimedes method according to the ISO standard 39231/1-1979(E) (12). In this way, the interconnected porosity (IP) of the fabricated scaffold was measured using the following equation:
where W1 is the dry weight of the scaffold, W2 is the weight of the scaffold after removing from water, and W3 is the weight of the scaffold after soaking in water.
3.3. Contact Angle Measurement of the Scaffold
The wettability property of the freeze-dried alginate scaffold was evaluated by the sessile drop technique using water contact angle measurement machine (GBX Instruments Co.). For this purpose, the silica substrate of the machine was washed with ethanol (Merck, Germany) before the beginning of each experiment. The fabricated scaffold was placed in the transparent chamber under the needle. Next, a distilled water droplet was placed on the surface of the scaffold gently. The shape of the formed distilled water droplet on the surface of the scaffold was monitored using a high-resolution time-lapse camera (Nikon D90, resolution: 13 megapixels). Image J software was used to analyze the droplet and measure the contact angle. The experiment was repeated three times for each concentration of the alginate scaffold.
3.4. Mechanical Strength of the Scaffold
The compressive mechanical strength of the fabricated alginate scaffold was evaluated by Instron 5542 mechanical tester (Norwood, MA, USA). Samples were placed between the grips of the Instron machine and compressed in the axial direction at the rate of 1 mm/min.
3.5. Evaluation of Morphology, Distribution, and Size of Pores
The morphology, distribution, and size of pores of the prepared scaffold were investigated by scanning electron microscopy (SEM, Philips XL30: Eindhoven, The Netherlands). The image analysis software (Image J 1.40 g) was used to estimate the average pore size of samples (by selecting five individual pores for each sample).
3.6. In Vitro Degradation Rate of the Prepared Scaffold
Blood plasma is a neutral environment containing inorganic ions such as Na+, Ca2+, and K+. Furthermore, organic compounds such as proteins and amino acids can be found in blood plasma. Therefore, the in vitro degradation rate of the prepared scaffold was evaluated in three different media: distilled water, Dulbecco’s Modified Eagle’s Medium (DMEM; Gibco, Grand Island, USA), and DMEM with 10 % Fetal Bovine Serum (FBS, Sigma Aldrich, USA). The detailed composition of the used DMEM and DMEM+FBS solutions are listed in Table 1. For this measurement, the scaffold was sliced into small pieces (10 mm× 10 mm × 2 mm); the slices were immersed in the solutions and placed in a shaking incubator at 37ºC. The samples were collected after four weeks and dried at ambient temperature for 24 h. The percentage of weight loss was measured by the following equation:
where W1 is the weight of the dried gel and W2 is the weight of the scaffold after four weeks of soaking in the solutions.
| Compound | DMEM | DMEM + FBS |
|---|---|---|
| CaCl2 | 1.8 | 1.8 |
| NaCl | 109.4 | 109.4 |
| KCl | 5.37 | 5.37 |
| NaHCO3 | 44.05 | 44.05 |
| NaH2PO4 | 1.04 | 1.04 |
| MgSO4 | 0.81 | 0.81 |
| Glucose | 5.5 | 5.5 |
| Amino acid | 11.01 | 11.01 |
| Fetal bovine serum | None | 10% |
3.7. Periodontal Ligament Stem Cells (PDLSCs) Isolation and Culture
To evaluate the cellular behavior of the fabricated scaffold, periodontal ligament stem cells (PDLSCs) were used. Human PDL cells were extracted from human teeth. For this reason, after washing teeth several times with phosphate-buffered saline (PBS), the PDL was scraped from the middle third of the root surface. Obtained tissues were washed several times with sterile PBS, crushed to small pieces, put in a 25-cm2 culture flask, and incubated at 37ºC in 5% CO2 atmosphere. After reaching 80% confluence, the cells were cultured in DMEM (Gibco, Grand Island, USA) supplemented with 10% (v/v) FBS (Gibco, Grand Island, USA), 100 unit/mL of penicillin, and 100 mg/mL of streptomycin in a humidified incubator at 37ºC with 5% CO2.
3.8. Cell Viability Assay
To evaluate the biocompatibility behavior of the optimized alginate scaffold, the samples were put in a 96-well plate, sterilized by ultraviolet light (254 nm) irradiation for 30 min in a laminar flow hood, and seeded with 1 × 104 cells/well. The cytotoxicity and the proliferation of the cells on the scaffold were assessed by 3-(4, 5-dimethylthiazol-2-yl)-2, 5-diphenyltetrazolium bromide (MTT) assay kits (Sigma-Aldrich, St. Louis, USA) according to the manufacturer’s instructions. The cells in the wells of the plate without scaffolds were treated as positive control. All experiments were performed in triplicate and the absorbance was read by an Awareness Technology Microplate-Reader.
3.9. PDLSCs Differentiation into Osteoblast-like Cells in the Fabricated Scaffold
A sterilized fabricated scaffold was placed in a 24-well plate and 2 × 104 cells were seeded in each well. Differentiation media containing DMEM/F12 + FBS 10%, dexamethasone 10 nM, ascorbic acid 50 μg/mL, and β glycerol phosphate 10 mM were added to each well for 21 days. The media were replaced by fresh ones every three days.
3.10. Real-Time RT-PCR
The effect of different groups of fabricated alginate scaffolds on the expression of four main bone correlated genes including osteopontin, collagen I, osteocalcin, and alkaline phosphatase (ALP) were evaluated by real-time reverse transcription (RT)-polymerase chain reaction (RT-PCR). After 21 days of PDLS cell culture on the surface of scaffolds, samples were washed using PBS. The total RNA was isolated using Qiazol reagent kit according to the manufacturer’s instruction. Next, cDNA was fabricated by M-MLV Reverse Transcriptase kits. The obtained cDNA was subjected to quantitative RT-PCR and gene expression was measured by SYBR-Green (TAKARA, USA). The used primer sequences for osteonectin, collagen I, osteocalcin, and ALP are presented in Table 2.
| Gene | Forward (5’-3’) | Reverse (5’-3’) |
|---|---|---|
| COL (Ι) | ATGGCTGCACGAGTCACACC | CAACGTCGAAGCCGAATTCC |
| OCN | TGCTTGTGACGAGGTATCAG | GTGACATCCATACTTGCAGG |
| ALP | CCTCGTTGACACCTGGAAG | CTGGTAGTTGTTGTGAGCATAG |
| OPN | GCCGACCAAGGAAAACTCACT | GGCACAGGTGATGCCTAGGA |
Abbreviations: ALP, alkaline phosphate; COL (I), collagen I; OCN, osteocalcin; OPN, osteopontin.
3.11. Immunofluorescence Analysis
After 21 days of PDLSCs induction, immunocytochemistry as double staining was used to evaluate the expression of specific markers for differentiation. After fixation with 4% PFA (Sigma-Aldrich), cells were permeabilized by 0.2% Triton-X100 (Sigma-Aldrich) for 30 min and then blocked with 5% bovine serum albumin in PBS. After 45 min, the cells were incubated overnight with primary antibody collagen I, mouse monoclonal AB (Abcam, 1:200), osteonectin, and Rabbit Monoclonal AB (Abcam, 1:200). A secondary antibody (Alexa Fluor@488 donkey anti-mouse IgG and Alexa Fluor@594 donkey anti-rabbit IgG at a 1:500 dilution; Abcam) was used at 37ºC for 1 h. Between each step, slides were washed with PBS and nuclei staining was performed using 4’,6-diamidino-2-phenylindole (DAPI, Sigma). Cells were examined by fluorescence microscopy (Olympus BX51, Japan).
3.12. Statistical Analysis
All experiments were repeated three times. The data are shown as means and standard error. Two-way ANOVA was used to evaluate the data. Significant differences were shown as *P < 0.05, **P < 0.01, and ***P < 0.001.
4. Results and Discussion
4.1. Morphology and Structure Characterization
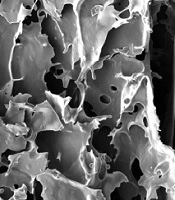
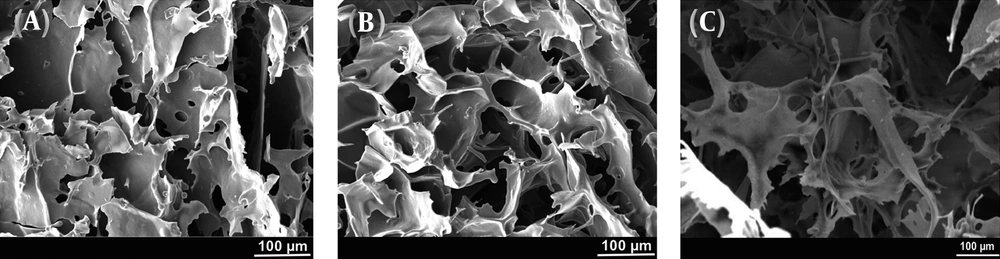
The SEM micrographs of the prepared scaffolds by the freeze-drying method in Figure 1 show the morphology, size, and interconnectivity of pores. The pore size analysis by Image-J software showed that the pore sizes of the 4%, 8%, and 16% (w/v) alginate scaffolds were in the range of 250 - 320 μm, 220 - 250 μm, and 180 - 200 μm, respectively. All samples showed pore interconnectivity. Flatley et al. (13) reported that using scaffolds with pore sizes of 200 - 500 μm is suitable for hard tissue regeneration. Hulbert et al. (14) showed that the minimum pore size for osteoconduction is 150 μm. Freyman et al. (15) concluded that the minimum pore size of scaffolds should be greater than 100 μm to allow for nutrient transferring among the pores. As shown in Figure 1, all the fabricated alginate scaffolds with different concentrations had suitable pore size structures for hard tissue replacement.
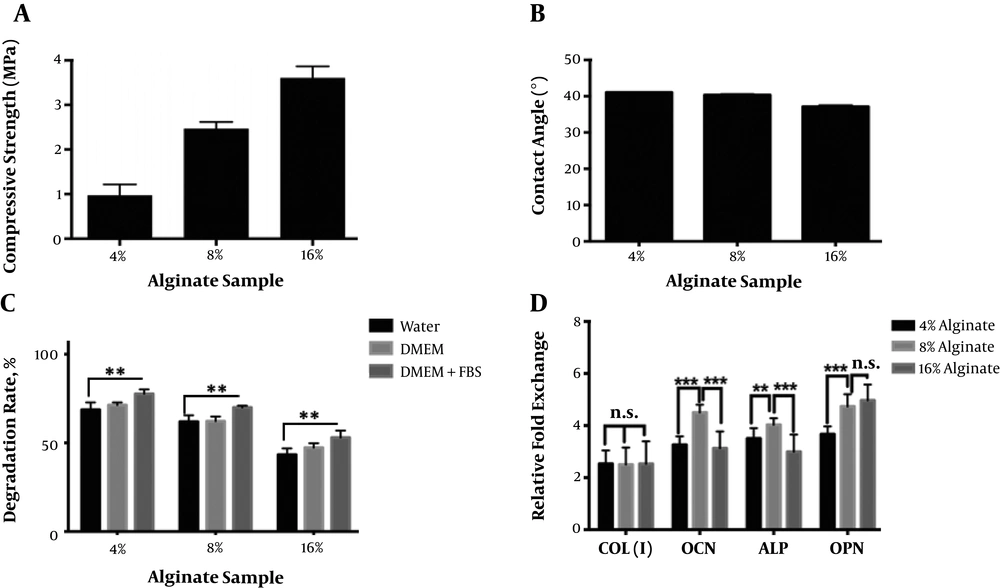
The compressive strength of the alginate scaffolds showed that the mechanical strength of the alginate scaffolds improved with increasing the alginate concentration (Figure 2A). The poor mechanical properties of the 4% alginate scaffold would limit its application in cartilage and bone tissue engineering.
(A) Compressive strength, (B) contact angle of water on the surface, (C) degradation rate, and (D) quantitative mRNA expression analysis of osteoblast-like cells derived from PDLS cells seeded onto samples of alginate scaffolds with different concentrations of 4%, 8%, and 16% (w/v) fabricated by the freeze-drying method (n.s. stands for not significant, *P < 0.05, **P < 0.01, and ***P < 0.001).
Contact angle analysis was conducted to evaluate the surface properties of different concentrations of the alginate scaffolds. Figure 2B shows the variation of water contact angle on the surface of fabricated scaffolds. As can be seen, the contact angle decreased from 41 ± 0.17º to 37 ± 0.56º as the concentration of alginate increased from 4 to 16% (w/v). However, all three samples showed hydrophilic surfaces that could facilitate cell adhesion and ingrowth.
4.2. Degradation Behavior of Prepared Scaffolds
The degradation trends of the prepared alginate scaffolds with different concentrations (4, 8, and 16% (w/v)) after 28 days of soaking in water, DMEM, and DMEM + FBS are shown in Figure 2C. As can be seen, the degradation rate was significantly higher in DMEM + FBS media for all the samples. A significant difference was observed between the degradation rates of samples soaked in water and in DMEM + FBS. After 28 days, the degradation rate of 4% alginate reached ~76% in DMEM + FBS, showing a weight loss of almost 1.5 times the 16% alginate soaked in the same solution. As can be seen, the degradation rate of 8% alginate was ~70% in DMEM + FBS.
One of the most important properties that a scaffold must full fill is its degradability over time (16). The present study demonstrated that the obtained 4% alginate scaffold exhibited high weight loss in different solutions and its dissolution rate was much higher than the rates of 8% and 16% alginate scaffolds throughout the degradation time and in all the examined solutions, indicating that the obtained 4% alginate scaffold had an unstable structure. The high dissolution rate of 4% alginate scaffold may be attributed to its high porosity volume compared to both 8% and 16% alginate scaffolds.
It was found that the 4%, 8%, and 16% alginate scaffolds had the interconnected porosities of about 83 ± 2.3, 80 ± 3.7, and 58 ± 4.3, respectively (Table 3). Based on these results, the 16% alginate scaffold had almost a closed-porosity structure and its low volume interconnected porosity could not meet the requirement for cartilage and bone tissue substitute.
| Sample | Interconnected Porosity | Total Porosity |
|---|---|---|
| Alginate 4% | 83 ± 2.3 | 85 ± 3.1 |
| Alginate 8% | 80 ± 3.7 | 83 ± 2.8 |
| Alginate 16% | 58 ± 4.3 | 80 ± 3.9 |
One of the most important properties of scaffolds in hard tissue engineering applications is their osteogenesis abilities. Here, we evaluated the effect of alginate concentration on gene expression levels using RT-PCR (Figure 2D). As can be seen, there was no significant difference in collagen type I expression by changing the concentration of alginate. In the case of osteocalcin, ALP, and osteopontin genes, the levels of gene expression of the cells exposed to the 8% alginate were significantly higher than the gene expression levels of the cells contacting the 4% alginate. Osteocalcin and ALP genes exhibited the highest expression in the 8% alginate scaffold (having the highest amount of interconnected porosity) and the lowest expression in the 16% alginate scaffold (having the lowest amount of interconnected porosity). Other researchers showed that different physical and chemical properties of scaffolds could significantly affect the gene expression (17). Interestingly, as can be seen in Figure 2D and Table 1, the gene expression level decreased dramatically with decreasing the pore interconnectivity.
Ideal scaffold biomaterials for use in bone and cartilage tissues should be satisfied with a normal level of biodegradability, high porosity, high osteogenetic ability, and good mechanical properties. In this study, we showed that 4% and 16% (w/v) alginate scaffolds had weak mechanical properties and poor osteogenesis abilities, respectively; thus, they could not be good candidates for use as hard tissues substitutes. Therefore, our proposed scaffold to use in hard tissue engineering applications is the alginate scaffold made with the concentration of 8% alginate (w/v).
Thus, we evaluated in-vitro PDLS cells seeded on the optimized 8% alginate scaffold at different time points and the results are presented in the following.
4.3. Cell Culture on the Optimized Alginate Scaffold
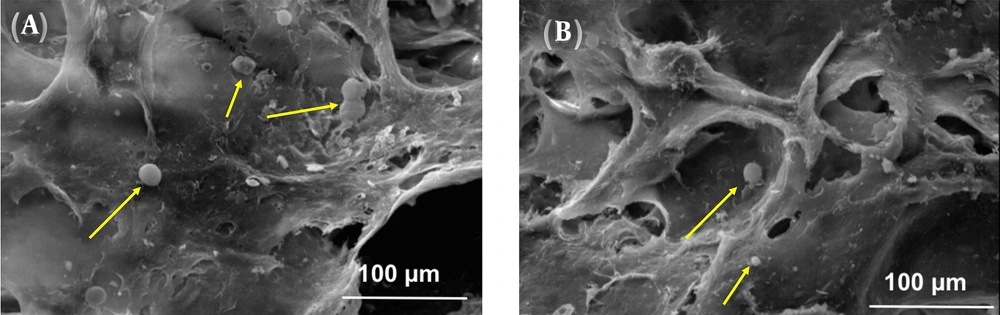
Figure 3 shows the morphology of PDLS cells cultured on the optimized 8% (w/v) alginate scaffolds for 9 days using SEM. As can be seen, the cells attached and grew on the surface of the scaffold over time. The figure demonstrates that cells attached and spread on the surface by normal spherical shapes and most of them showed several extensions (marked by yellow arrows). The attachment and growth of cells on the surface and in the pores of a scaffold are the most important properties of a completely safe and biocompatible scaffold (18). Cells attachment and spread on the surface of the optimized 8% (w/v) alginate scaffold are obvious in Figure 3.
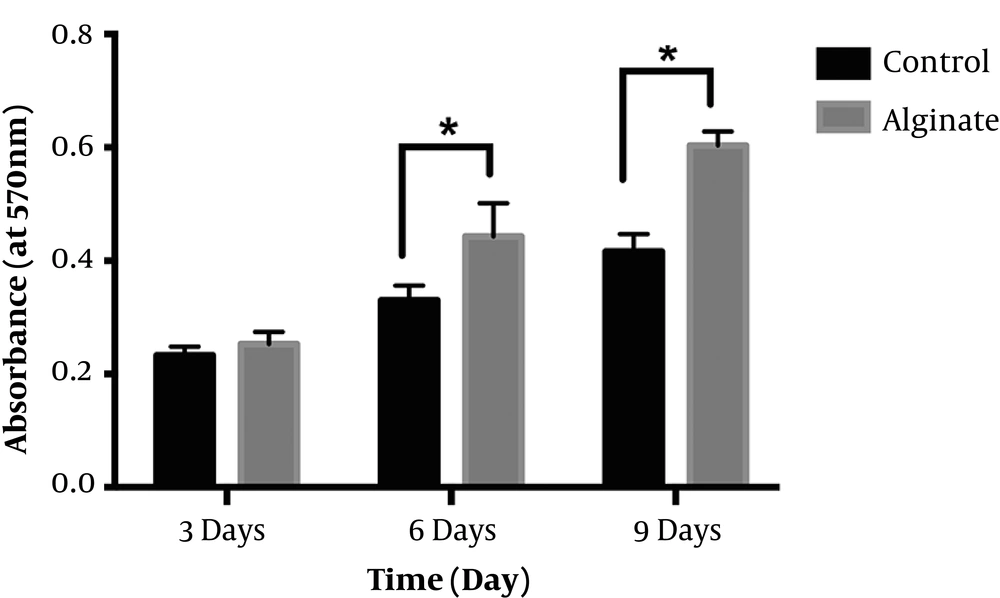
Figure 4 shows the results of MTT assay of the optimized 8% (w/v) alginate scaffold and a control sample (only cells + media) after 3, 6, and 9 days of cell culture. The results showed that after 3 days of culture, there was no significant difference between control and alginate samples. However, cells continued to grow over time. By extending the culture duration up to 6 days, the optical density of the optimized 8% (w/v) alginate scaffold significantly increased compared to the control sample (P < 0.05). As can be seen, the synthesized alginate sample not only did not have any negative effect on PDLS cells, but also could promote cell ingrowth. These results strongly show that the optimized alginate scaffold was completely safe, with cell-friendly behavior and no cytotoxic effect.
4.4. Differentiation of PDLSCs to Osteoblast on the Optimized 8% (w/v) Alginate Scaffold
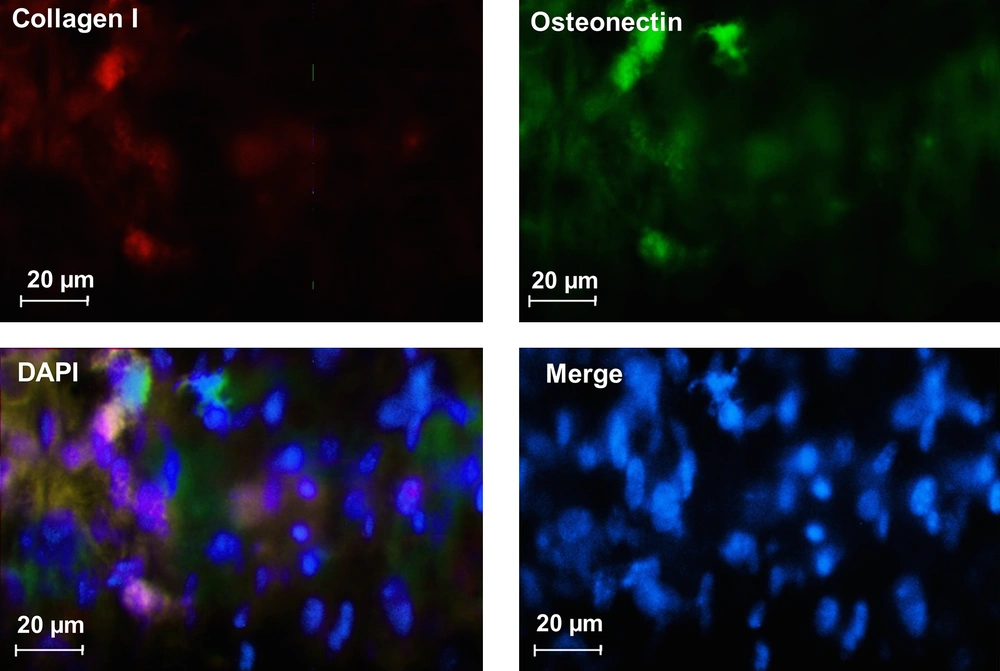
Figure 5 shows the immunocytochemistry staining for collagen I and osteonectin related to osteoblast-like cells. This figure shows that PDLSCs differentiated to osteoblast-like cells successfully during culture of PDLSCs in differentiation media. These results show that the fabricated modified alginate scaffold was osteoconductive and could be used in hard tissue engineering applications.
Alginate-based scaffolds are promising biomaterials in cartilage and bone substitute applications. However, their mechanical, physical, and osteogenesis behaviors should be tailored for a successful replacement. In this study, we showed that the 8% alginate scaffold had the optimized concentration to meet the requirement of hard tissue engineering applications.
4.5. Conclusions
In this study, we modified the mechanical properties, surface characteristics, porosity structure, and cell behavior of alginate scaffolds fabricated by the freeze-drying method only by changing the concentration of alginate. The modified 8% (w/v) alginate scaffold had interconnected porosity of more than 80%, the compressive strength of ~2.6 MPa, and the contact angle of water on the surface of 37º ± 0.56. The results showed that the modified alginate scaffold exhibited high cell viability and could significantly promote osteoblast-like cell proliferation. Our results confirmed the differentiation of periodontal ligament stem cells into osteoblasts by using the optimized alginate scaffold. The results support the use of 8% (w/v) alginate scaffolds fabricated by the freeze-drying method as bone and cartilage tissue substitutes.