1. Background
Alzheimer’s is a multifactorial disorder that is affected by aging, genetic risk factors, reactive oxygen species and inflammation. Studies have shown that this disease initiates with synaptic loss, neuronal impairment, proliferation of microglial cells and is followed by inflammation (1, 2).
The exact mechanism of Alzheimer’s disease (AD) is not completely understood; however, plaque deposition by fibrillar aggregates of amyloid beta peptide (Aβ) is recognized as a factor in the pathology of this disorder and is measured in AD pathogenesis (2, 3).
It has been documented that inflammation induced by Aβ results in memory loss, oxidative stress and an increase in TNF-α (2, 3). Furthermore, increased oxidative stress in the brain is accompanied by memory and cognitive loss and development of AD (2). Elevated oxidative stress causes formation of senile plaque, amyloid deposits and synaptic loss that result in loss of the structure and function of neurons. One vital contributing factor for management and prevention of neurodegeneration is the control of inflammatory factors and oxidative stress (1, 2).
Management of AD has focused on decreasing the formation of Aβ by normalizing cholinergic function with the administration of cholinesterase inhibitors, non-steroidal anti-inflammatory drugs and by reducing neuro-inflammation medications such as COX-2 inhibitors. Most of these medicines have side effects. In this context, natural herbal sources with beneficial health effects and few side effects can be a good alternative for the prevention or management of inflammation and oxidative stress in neurodegenerative diseases (1, 2).
Virgin coconut oil (VCO) is useful edible oil that has been shown to influence inflammatory disorders. Vysakh et al. (4) showed that VCO significantly inhibits inflammatory mediators in chronic arthritis. This type of oil can normalize oxidative stress and inhibit lipid peroxidation (5).
2. Objectives
The current study investigated the neuroprotective properties of VCO in Aβ-induced Alzheimer’s rats as a potential antioxidant and anti-inflammatory agent. The antioxidant effects and TPC of VCO in the in vitro condition were determined as well.
3. Methods
3.1. VCO Preparation (Fermentation Method)
The VCO under study was produced according to the previous studies (6).
3.2. Total Phenolic Content and Total Flavonoid Content
The total phenolic content (TPC) in the VCO was measured with Folin-Ciocalteau reagent using a previously published method and Gallic acid as the standard (7). The total flavonoid content (TFC) content of the oil was measured using the AlCl3 colorimetric assay. Quercetin was used as the standard.
3.3. DPPH Radical Scavenging Activity Assay
This method was performed according to published recommendations using 1,1-diphenyl-2-picrylhydrazyl (DPPH) assay by adding 1 mL of 1 mM DPPH solution to 3 mL of VCO solution (in methanol; 25 - 100 mg/L. Butylated hydroxytoluene (BHT) was used as the reference (7).
3.4. Ferric Reducing Antioxidant Power
The ferric reducing antioxidant power (FRAP) was used to determine the total antioxidant activity of the method described previously with small modifications. FRAP is based on the ability of the antioxidant to reduce Fe3+ to Fe2+ (7).
3.5. Animals
Male Wistar rats (8 week) were purchased from Pasteur Institute of Iran. All animals were maintained at room temperature (22 ± 1°C) in a 12 hours light/dark cycle. All procedures were done according to the guidelines of the NIH Guide for the Care and Use of Laboratory Animals. After about 7 days of adaptation, the rats were randomly divided into six groups (n = 10/group); 1: control, 2: sham surgery, 3: sham surgery receiving normal saline; 4: Alzheimer’s rats (induced with Aβ 1 - 40); 5: Alzheimer’s rats + 8% VCO; 6: Alzheimer’s rats + 10% VCO.
3.6. Aβ (1 - 40) Injection and Drug Treatments
Aβ (1 - 40) peptide (Tocris Bioscience; UK) was dissolved PBS (10 µg in 10 µL of sterile PBS) and incubated at 37°C for 7 days to induce peptide aggregation. The aggregated Aβ and vehicle was injected slowly over 5 minutes into the left lateral ventricle of all animals in groups 4 to 6 as described in a previous study (8). One week after Aβ injection, 10% VCO and 8% VCO and were administered for 8 weeks.
3.7. Preparation of Hippocampus Homogenates
The hippocampus was homogenized in lysis buffer (Sigma-Aldrich; USA). All factors are presented as mg of protein of the hippocampus as determined by the Bradford method.
3.8. Catalase and Glutathione Peroxidase Activity
The Catalase (CAT) and glutathione peroxidase (GPx) activity were determined using commercially available kits (BioLegend; UK) according to manufacturer instructions.
3.9. Superoxide Dismutase Activity
The superoxide dismutase (SOD) activity in the hippocampus homogenate and serum were measured using a Randox kit.
3.10. Acetylcholinesterase Activity
The activity was measured by determining the yellow anion formation from the reaction of the thiocholine generated by enzymatic hydrolysis of ATCh and Ellman's reagent. Briefly, ATCh (as a substrate, 75 mM), PBS (pH 8.0, 0.1 M), and 5,5’-dithiobis(2-nitrobenzoic acid) (Ellman's reagent, 10 mM) were mixed at a ratio of 2:150:5. The change in absorbance was measured at 412 nm for 3 minutes at regular intervals of 30 seconds using a UV-visible spectrophotometer immediately after the enzyme was obtained (10 µL) (9).
3.11. TNF-α Level
TNF-α protein levels were determined using the rat TNF-α by enzyme-linked immunosorbent assay (ELISA) using commercial available kit (BioLegend).
3.12. Nitric Oxide Levels
The concentrations of nitric oxide (NO) in the hippocampus homogenates measured according to previous published paper (10).
3.13. Real-Time Polymerase Chain Reaction
Gene expression of TNF-α and iNOS mRNA was measured by real-time polymerase chain reaction (PCR). The total RNA was isolated from the hippocampus tissue using RNX-plus solution (Cinnagen; Iran). For cDNA synthesis, 2 µg of total RNA was reverse transcribed by using a cDNA reverse transcription kit (Thermo Scientific; Lithuania) (11). Gene expression assays were determined using real-time PCR (Roche; Germany) using Takara SYBR Master Mix (Shiga; Japan). The TNF-α primer sequences used were TNF-α (forward): TGTTCATCCGTTCTCTACCCA, TNF-α (reverse): CACTACTTCAGCGTCTCGT, iNOS (forward): AGAGACGCTTCTGAGGTTC, iNOS (reverse): TTGATGCTTGTGACTCTTAGG and GAPDH (housekeeping control, forward): AAGGTCGGTGTGAACGGATTTGG, GAPDH (reverse): TCCTGGAAGATGGTGA TGGGTT. The products of each gene were separated on 1% agarose gel and visualized using SYBER safe staining (10).
3.14. Histopathological Changes
The brain tissue was fixed by immersion in 4% paraformaldehyde solution. Hematoxylin-eosin (H&E), Imonohistochemistry (anti-tau ser404; Abcam) and Congo Red staning were done. The images were quantified in Image J software.
3.15. Statistical Analysis
All statistical analysis was done in SPSS software (version 20). The results from the histological counts are presented as percentage of control, whereas the data from the biochemical factors are expressed as mean ± SD. One-way analysis of variance (ANOVA) and Tukey’s post-test were used to compare the groups. A P value of less than 0.05 was considered significant.
4. Results
4.1. Total Phenolic and Flavonoids Contents
The TPC and TFC of the VCO were 60 mg GAE/100 g and 19 mg/100 g oil, respectively.
4.2. In Vitro Antioxidant Activity
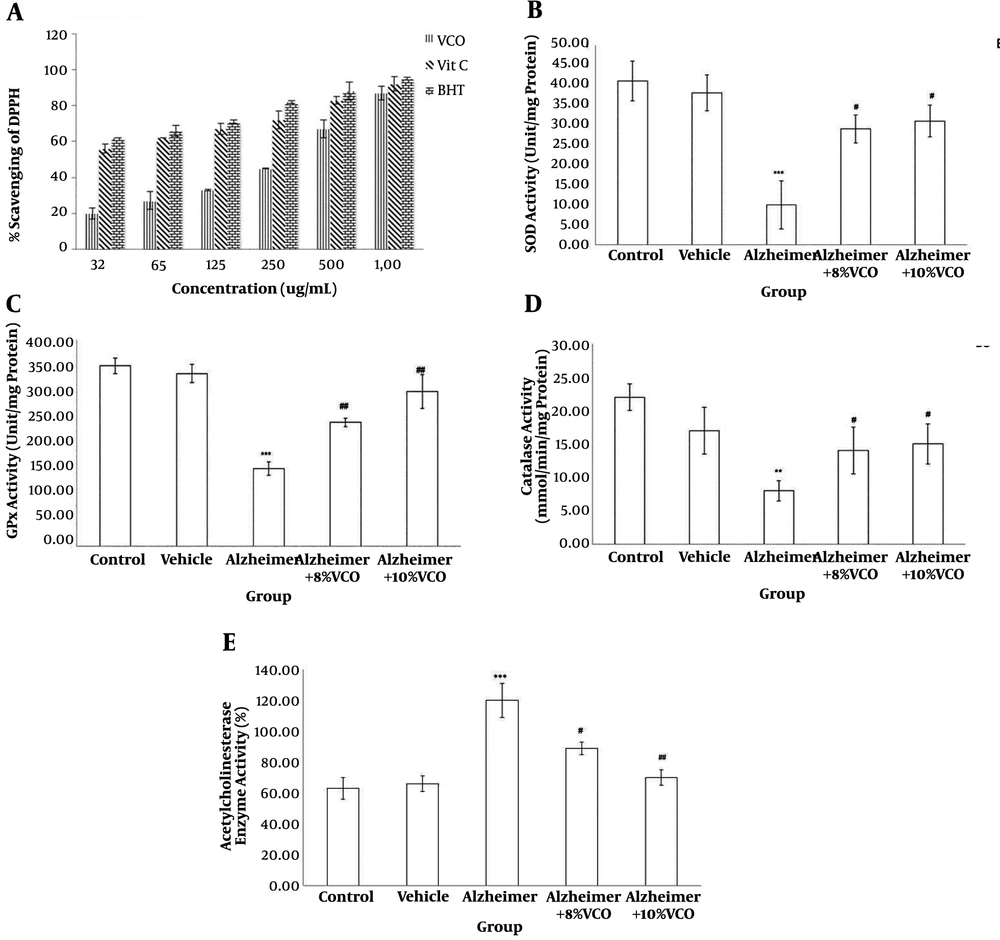
The VCO exhibited potential DPPH radical scavenging activity (Figure 1A). At a 1 mm concentration, VCO showed substantial scavenging activity, similar to ascorbic acid and BHT. The significant antioxidant activity of VCO was determined by the FRAP method. VCO at a dose of 1 mg/mL had a FRAP value of 2.11 mmol/L, which is equivalent to FeSO4.7H2O and ascorbic acid showed 2.50 mmol/L.
DPPH (1, 1-diphenyl-2-picrylhydrazyl) scavenging activity (A) of VCO. Levels of superoxide dismutase (SOD) (B), glutathione peroxidase (GPx) (C) and Catalase (D), and percentage of acetyl cholinesterase enzyme activity (E) in the studied groups. Results are presented as the mean ± SD (P < 0.05). ***P < 0.001, **P < 0.01 and compared with control group. ##P < 0. 01 and #P < 0.05 compared with Alzheimer group.
4.3. Superoxide Dismutase, Catalase and Glutathione Peroxidase Activity
Aβ significantly induced oxidative stress in the brain. SOD (P < 0.001), CAT (P < 0.01) and GPx (P < 0.001) activity decreased significantly in the Alzheimer’s group (Figure 1B - D). VCO had a significant effect on the treated rats. It elevated the SOD, CAT and GPx levels in the brain (P < 0.05).
4.4. Activity of AChE in the Hippocampus
In this experiment, AChE activity in the brain as a cholinergic marker increased significantly in the animals receiving Aβ 1 - 40 (P < 0.001). Supplementation with 8% VCO (P < 0.05) and 10% VCO (P < 0.01) markedly attenuated AChE activity (Figure 1E).
4.5. TNF-α Protein and Gene Expression
In the hippocampus, the TNF-α level increased in the Alzheimer’s group and was decreased by VCO. Intracellular TNF-α expression (Figure 2A) was markedly elevated in the Alzheimer’s group compared to the control group (P < 0.001). The VCO-treated animals showed a significant decrease in TNF-α expression level (P < 0.05). The TNF-α protein levels (Figure 2C) decreased significantly with the administration of 10% VCO (P < 0.01).
Fold change of TNF-α (A) and iNOS (B) gene expression (normalized for glyceraldehyde 3-phosphate dehydrogenase [GAPDH]), TNF-α protein concentration (C) and hippocampus NO level (D) in the studied groups. Results are presented as the mean ± SD (P < 0.05). ***P < 0.001 compared with control group. ##P < 0.01 and #P < 0.05 compared with Alzheimer group.
4.6. iNOS Gene Expression and Nitric Oxide Levels
The effect of VCO on the iNOS gene expression (Figure 2B) and NO level (Figure 2D) in the hippocampus were investigated. The results showed that iNOS gene expression (P < 0.001) and NO levels increased significantly in the Alzheimer’s group. Increased doses of VCO markedly decreased both iNOS (P < 0.05) gene expression and NO (P < 0.01).
4.7. Virgin Coconut Oil Reduced Amyloid Beta Plaque
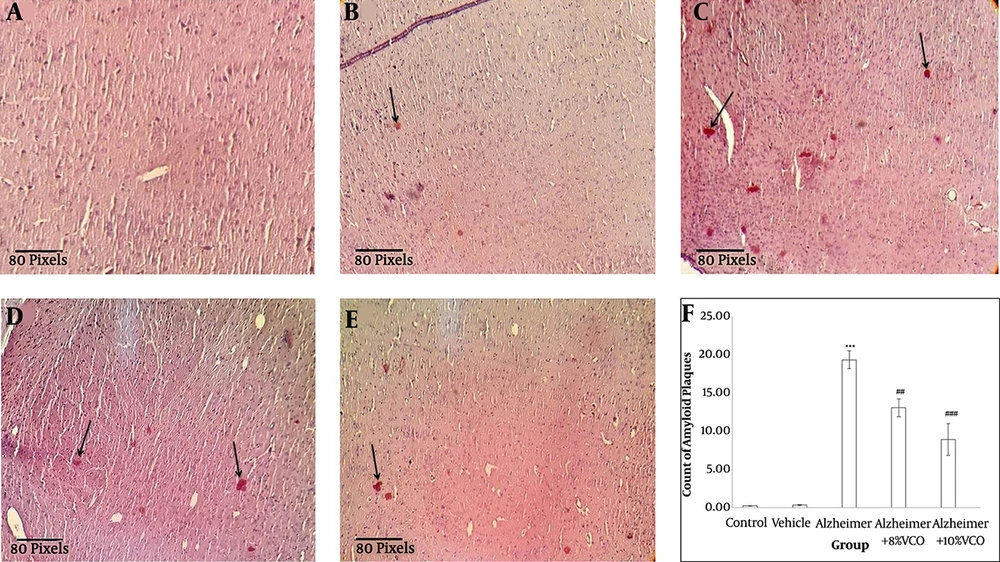
Figure 3 shows that the accumulation of Aβ peptides in the hippocampus are a major factor in the progress of AD pathogenesis (1). Congo red staining was used to determine the Aβ peptide accumulation. Aβ1-40 injection into normal animals resulted in significant Aβ peptide accumulation (P < 0.001). VCO treatment markedly reduced Aβ plaque in the test rats (8% VCO; P < 0.05 and 10% VCO; P < 0.01) compared to those in the AD group.
Brain histology after Congo Red staining. Staining the Congo Red was done to confirm the presence of amyloid plaque in the brain tissue (straight arrows) from control (A), vehicle (B), Alzheimer (C), Alzheimer + 8%VCO (D) and Alzheimer + 10% VCO (E) groups. Representative images of six sections per cortex and five brains per group were observed with × 20 objectives. Results are presented as the mean ± SD. ***P < 0.001, compared with control rats. ###P < 0.001 and ##P < 0.05 compared with Alzheimer rats.
4.8.Virgin Coconut Oil Reduced Positive Phosphorylated Tau Cell Count
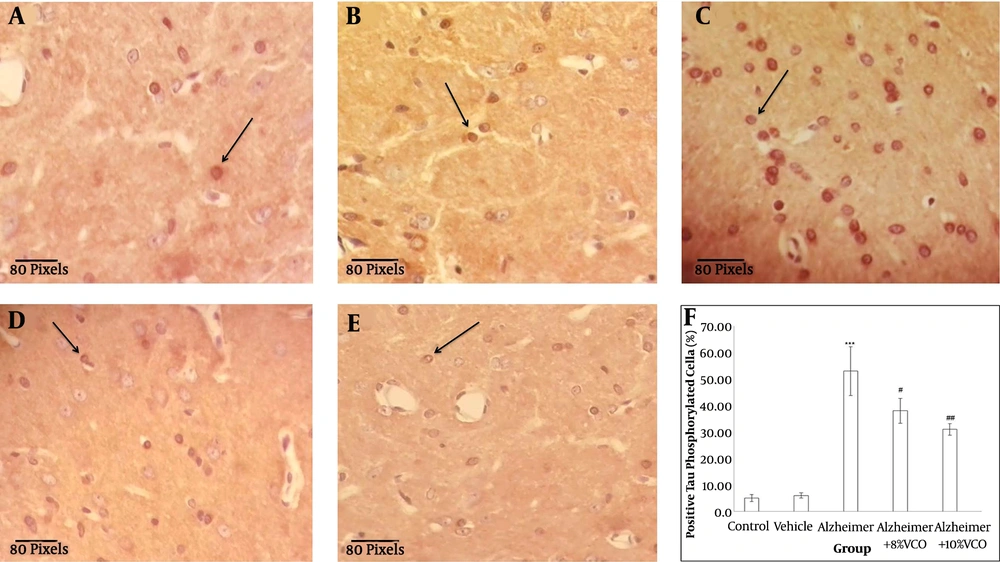
The presence of positive phosphorylated tau cells points to the formation of neurofibrillary tangles. The number of neurofibrillary tangles has a direct relation with the severity of AD. In this study, Aβ1-40 injection significantly increased the number of neurofibrillary tangles (P < 0.001) compared to normal animals. The administration of VCO reduced the number of positive cells (8% VCO; P < 0.05 and 10% VCO; P < 0.01) (Figure 4).
Representative IHC stained brain sections from control (A), vehicle (B), Alzheimer (C), Alzheimer + 8% VCO (D), Alzheimer + 10% VCO (E) groups. Representative images of six sections per cortex and five brains per group were observed with × 40 objectives. The positive tau phosphorilated cells (straight arrows), was shown. Results are presented as the mean ± SD. ***P < 0.001 compared with control rats. ##P < 0.001 and #P < 0.05 compared with Alzheimer rats.
4.9. H&E Staining Histological Results
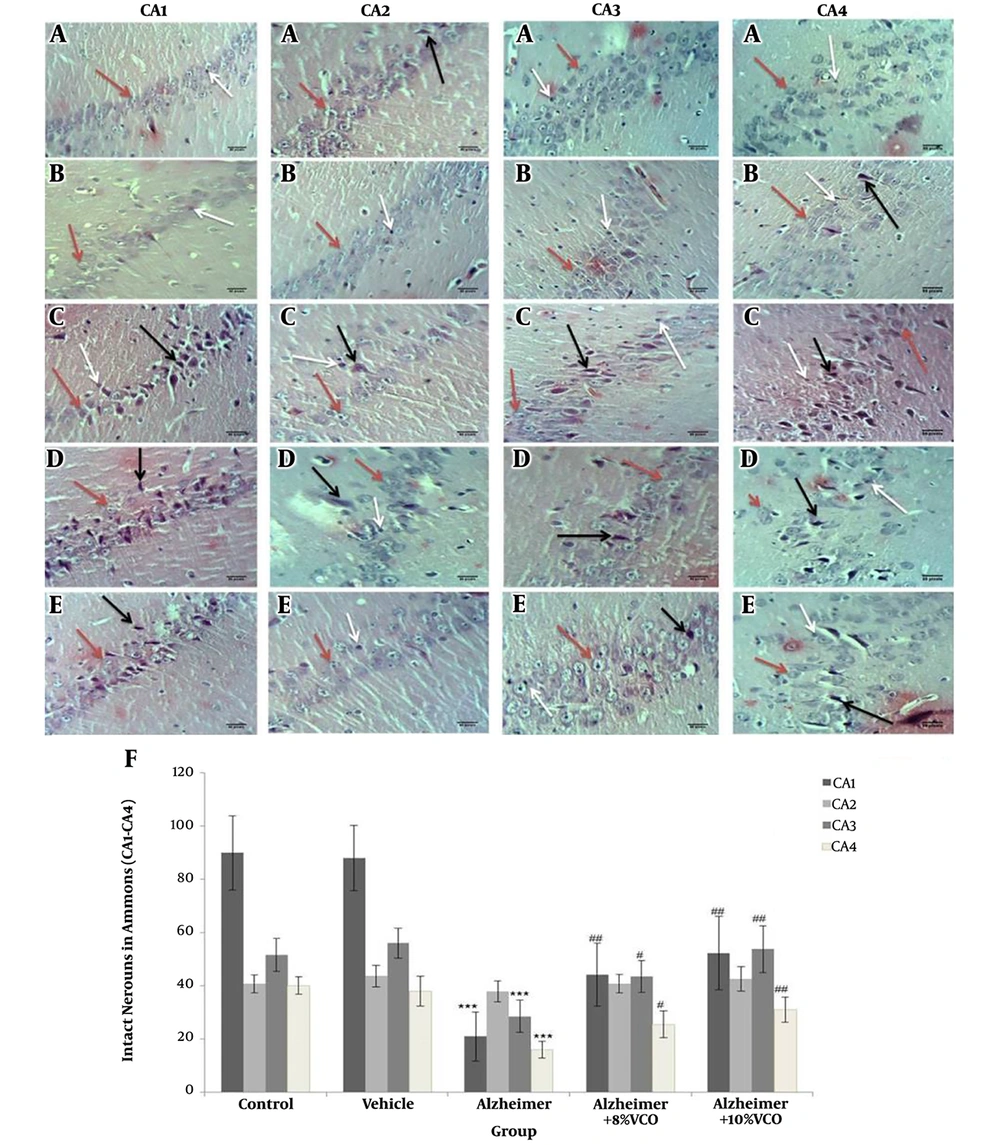
Figure 5 shows the morphological changes in various areas of the hippocampus. The criteria used to determine damage or cell death were: darkened and small cells (cells with spots and dark membranes) and autolysis (cells showing unusual nuclear morphology). To assess the results, the number of normal and abnormal neurons were determined and the intact neurons were counted in the CA. The results showed that intact cell count decreased significantly in CA1, 3 and 4, but the change in CA2 was not significant.
Representative H&E stained brain sections from control (A), vehicle (B), Alzheimer (C), Alzheimer + 8% VCO (D), Alzheimer + 10% VCO (E) groups. Representative images of six sections per hippocampus Ammon horn regions and five brains per group were observed with × 40 objective. The intact cells (red arrows), Autolysis (black arrows) and Darkened and small cells (withe arrows) was shown. Results are presented as the mean ± SD. ***P < 0.001 compared with control rats. ##P < 0.001 and #P< 0.05 compared with Alzheimer rats.
5. Discussion
The neuropathology of AD is complex and includes cerebral Aβ plaque accumulation, phosphorylated tau level, inflammatory reaction, increase in oxidative stress and change in AChE activity (1, 2, 12).
Because AD progresses along several pathological or neurotoxic pathways, natural medicines with multitarget and multifunctional features can have strong nutraceutical and pharmaceutical influences on AD (3). In this experiment, VCO exhibited potential nutraceutical and pharmaceutical effects through multitargeted pathways in Alzheimer’s animal models.
In the present study, TNF-α gene expression and levels increased markedly after Aβ injection. Treatment with both doses of VCO reduced inflammation in the hippocampus of the AD group. Previous studies have also found that elevated inflammatory cytokines participate in the progression of AD (13). It also has been established that an increase in inflammatory factors, especially TNF-α, can increase disease progression because of its ability to induce neuronal Aβ production and facilitate amyloid accumulation (13, 14).
The results of this study showed that amyloid accumulation significantly reduced by VCO in the hippocampus according Congo red staining. Change of structure and function of hippocampus is known one of the earliest detectable traits of AD and is progressively documented as a main component of early AD pathology (9, 12).
The results of the AD model using normal animals is in agreement with previous studies in showing that oxidative stress significantly increased in the rats. This can produce damage and death cell, along with Aβ accumulation, which is what occurs in AD (2). Furthermore, VCO supplementation significantly decreased oxidative stress markers in vitro and in vivo. It has been established that brain is more vulnerable to oxidative stress, probably because of the richness of iron, the high concentration of polyunsaturated fatty acids, the high rate of oxygen consumption and low antioxidant level.
Antioxidant enzymes such as SOD, CAT and GPx form the main defense mechanisms against ROS (7, 15, 16). It has been established that an increase in oxidative stress markers and a significant decrease in antioxidant activity occurs the brain of AD subjects (15).
The results of the present study shows that the level of CAT, SOD and GPx decreased significantly in the Alzheimer’s group and that treatment with VCO markedly normalized these markers. Because reduced antioxidant capacity is a part of normal aging and initiates very early in AD progression, natural antioxidants and free radical scavengers still hold promise for the management and prevention of various disease especially AD (17, 18).
The current findings showed that AChE activity increased significantly in the AD group, whereas treatment with VCO significantly normalized it activity (19). It has been reported that Aβ accumulation decreases the AChE concentration in the AD brain by elevating AChE activity. Although cholinesterase inhibitors and similar medications are administrated to alleviate AD symptoms, most are toxic and have undesirable side effects (17). Previous studies have shown that VCO is very safe and has numerous beneficial health effects (4, 5).
Previous studies have documented that synthesis of iNOS increased significantly after Aβ injection (20). This process may lead to a strong rise in the NO level in the hippocampus. It has been reported that iNOS/NO contributes to Aβ-induced neurotoxicity and may play a role in the pathogenesis of AD (21). In the current study, Aβ-stimulated iNOS production, but this was reversed by VCO. Interestingly, VCO normalized both iNOS expression and Aβ accumulation. In agreement with this findings, Tran et al. reported that NO overproduction participates in Aβ-induced brain injury and injection of Aβ1-40 increased iNOS mRNA expression (20).
5.1. Conclusions
This study initially established that VCO treatment strongly attenuates damage to nerve cells. It was shown that TNF-α, iNOS, AChE and oxidative stress decreased markedly in the VCO-treated AD rats compared to the AD rats. These findings strongly suggest that VCO can potentially attenuate damage to nerve cells and improve AD pathogenesis by suppressing the inflammatory response.
![Fold change of TNF-α (A) and iNOS (B) gene expression (normalized for glyceraldehyde 3-phosphate dehydrogenase [GAPDH]), TNF-α protein concentration (C) and hippocampus NO level (D) in the studied groups. Results are presented as the mean ± SD (P < 0.05). <sup>***</sup>P < 0.001 compared with control group. <sup>##</sup>P < 0.01 and <sup>#</sup>P < 0.05 compared with Alzheimer group. Fold change of TNF-α (A) and iNOS (B) gene expression (normalized for glyceraldehyde 3-phosphate dehydrogenase [GAPDH]), TNF-α protein concentration (C) and hippocampus NO level (D) in the studied groups. Results are presented as the mean ± SD (P < 0.05). <sup>***</sup>P < 0.001 compared with control group. <sup>##</sup>P < 0.01 and <sup>#</sup>P < 0.05 compared with Alzheimer group.](https://services.brieflands.com/cdn/serve/3170b/d6027285f81d03fb3483e688094165064b146a72/ans-85715-i002-F2-preview.webp)