1. Background
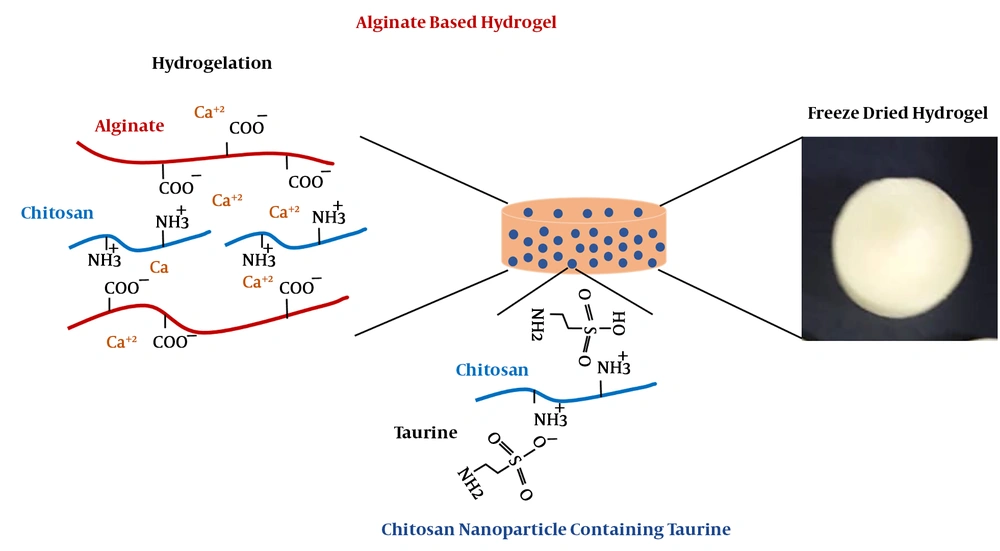
Hybrid hydrogels are recognized as worthwhile substrates, which are extensively used in biopharmaceutical and biomedical applications due to their profound properties such as low mechanical irritation of surrounding cells, controllable biodegradability, and biocompatibility in vitro and in vivo (1, 2). A functional hydrogel-based scaffold plays a critical role in cell attachment, growth, and tissue fabrication as well as released drug (1-3). Various composite hydrogels have been prepared from inert, inactive, and bioactive substrates in multiple fields of biomedical applications due to biocompatibility and biodegradation. Furthermore, inorganic materials with bioactive properties are utilized to direct cells toward specific target cells and tissues (4, 5). Among these substrates, alginate, a polyelectrolyte with negative charges on its backbone, has been widely used in tissue engineering and regenerative medicine due to its excellent biocompatibility, relatively low cost, and easy gelation in variable ways of crosslinking systems. Alginate-based hydrogel could form three dimensional network through anionic crosslinking reactions with divalent cations such as Ca2+, as well as chemical crosslinking, including enzymatic or photo-initiation approaches (4-6). In addition, chitosan is a linear cationic polysaccharide composed of randomly distributed β-(1-4)-linked D-glucosamine and N-acetyl-D-glucosamine residues. In general, chitosan is obtained from the thermochemical deacetylation of chitin in the presence of alkali or from certain fungi naturally (4, 6). It has been widely used in pharmaceutical and biomedical applications, due to its biological properties such as biocompatibility, low toxicity, and good characteristics for cell culture systems (6-10). Moreover, chitosan could be crosslinked with negatively charged molecules through electrostatic interactions such as sulfur-containing amino acids, glycosaminoglycans, proteoglycans, and growth factors (10, 11). These properties allow chitosan to bound and retain bioactive molecules on its structure (10, 11). Chitosan nanoparticle is produced by exploiting different techniques via ultrasonication or ionic gelation (6, 12). Ultrasonication arises from acoustic cavitation phenomenon, which generates strong shear forces through the generation of rapid solvent molecule streaming, which leads to cavitation of bubbles and raises shock waves during bubble collapse (12). The shear forces cut 1, 4-glycosidic bonds of chitosan that is aggregated; consequently, the size of particles is reduced in nano-dimension scale (12-14). On the other hand, taurine, a non-protein amino acid containing sulfur, is found in biological fluids and tissues and is the end product of cysteine metabolism in the body (12-14). Taurine has been implicated various beneficial physiological functions and biomedical actions, including anti-oxidation, neuromodulation, membrane stabilization, as well as the regulation of calcium homeostasis and metabolism of lipids. Introduction of such a sulfur-containing group to the molecule of chitosan is performed either by sulfoalkylation that results in the N, O-substituted products or direct sulfonation such as regioselective reaction (11, 13, 14). In this study, we have attempted to encapsulate taurine within nano-structured chitosan followed by ionic interaction of oppositely charged molecules to provide a suitable substrate for cell culture systems and drug delivery applications (Figure 1).
We first demonstrate the possibility of obtaining taurine-loaded chitosan nanoparticle through the electrostatic interaction of taurine and chitosan and ionic gelation technique. Here, we specifically focused on the tunability of the hydrogelation manner and characteristics of the resultant hydrogel. The taurine was loaded into chitosan nanoparticle composed with alginate gelated through divalent Ca2+cation, then cytocompatibility of this hybrid hydrogel was evaluated for tissue engineering and drug delivery applications.
2. Methods
2.1. Materials
The alginic acid polymer (molecular weight (MW): 70 kDa) was purchased from Qingdao Huanghai Biological Pharmaceutical Co., Ltd (Qingdao, PR China). The chitosan (MW: 100-150 kDa; the degree of deacetylation, 85%) was also purchased from Haixin Biological Product Co., Ltd (Ningbo, PR China). Tripolyphosphate (TPP) and calcium chloride were obtained from Sigma-Aldrich (St. Louis, MO). Dulbecco’s modified Eagle’s medium (DMEM) and fetal bovine serum (FBS) were purchased from Gibco Technologies (Logan, UT). All other chemicals in analytical grade were obtained from a variety of vendors.
2.2. Endometrial Stem Cell Isolation and Characterization
Endometrial stem cells (EnSCs) were collected after obtaining the patient's written informed consent, according to the Ethics Committee of Research of Tehran University of Medical Sciences by the method of Ebrahimi-Barough et al. (15). In brief, endometrial biopsies were obtained from donors using endometrial sampling device and maintained in Hanks balanced salt solution containing 10% (v/v) FBS, and 1%penicillin/streptomycin (P/S; Hyclone). The tissues were washed several times with phosphate buffered saline (PBS) and enzymatically disintegrated with collagenase type I (Sigma) under continuous agitation for 45 min. The resulting suspension was neutralized with cultured medium and passed through 40 μm cell strainer to separate the epithelial and stromal cells. Then the cell suspension was washed and centrifuged to separate the supernatant from the pellet at 1500 rpm for 5 min. Subsequently, the obtained cells were seeded in polystyrene tissue culture dish (PS). After 24 h, non-adherent cells were removed by changing the medium. When adherent cells reached 80% of confluence, those were harvested from the plates with 0.25% trypsin-EDTA solution and replated for three to five subculture.
2.3. Preparation of Chitosan Nanoparticle and Characterization
Nano-structured chitosan powder was produced using ionic crosslinker and ionotropic gelation technique (12). To this aim, the solution of chitosan 2 mg/mL in acetic acid buffer 0.1 M was prepared and in the following, the taurine 4 mM was dissolved in chitosan solution. For ionic gelation, the dissolved 1 mg/mL TPP in water was added to chitosan solution (TPP: chitosan volume ratio was 1:7) in rate of 20 μL/min using a syringe pump (SP1000, Fana-varan Nano-Meghyas, Tehran, Iran) while under constant magnetic stirring at 40°C for 45 min. The particles then were removed via centrifugation at 14000 rpm for 40 min and washed with distilled water three times. The fabricated nanoparticles were lyophilized and stored at 4°C for further use.
2.4. Hydrogel Preparation
Calcium-alginate hydrogel derivatives tested in this study were prepared by dropping aqueous alginate into a calcium chloride solution. Sodium alginate was dissolved in Krebs-Ringer-Hepes-bicarbonate (KRH, pH: 7.4) buffer at distinct compositions of 2% (w/v) and 3% (w/v) and poured in well plate. The calcium chloride solution 200 mM containing 0.1% (w/v) synthesized chitosan nanoparticle were dropped into 1 mL alginate solution at a ratio of 10:1 (v/v) and formed instantaneously gel in the shape of the pill. The prepared gels were rinsed with distilled water several times to remove non-crosslinked calcium chloride and chitosan nanoparticles.
2.5. Scaffold Characterization
Physical specification of chitosan nanoparticle including particle size, polydispersity and zeta potential were measured using a dynamic light scattering (DLS). Hence, the samples were diluted in distilled water with a viscosity of 1.0 cP at a temperature of 25°C. The amount of encapsulated taurine in nanoparticle was obtained spectrometrically (UV-VIS) at 570 nm after particles centrifugation by the use of a ninhydrin reagent. Loading efficiency (%LF) and encapsulation efficiency (%EE) of taurine in chitosan nanoparticle were determined according to the below formula.
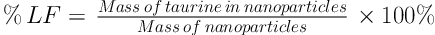
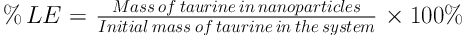
The composition of hydrogel was evaluated by Fourier transform infrared spectroscopy (FTIR; Nicolet NEXUS 670, Thermo Scientific, Waltham, MA, USA). The IR spectra of hydrogel, in transmittance mode, was obtained in the spectral region of 400-4000 cm-1 using a resolution of 4 cm-1. Scanning electron microscopy (SEM; AIS2100, Seron Technology, Korea) was utilized to observe the surface morphology and pore morphology of the hydrogel. Thus specimens were sputter-coated with gold (60 mA current, and at an accelerating voltage of 25 kV and duration of 180 s). The porosity of the hydrogel was determined via liquid displacement method and using the below equation. In this equation V1 is considered an initial volume of 96% ethanol, V2 is its volume after hydrogel soaking in ethanol (as ethanol filled the pore space), and V3 is the volume of the ethanol after the removal.
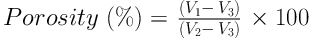
The hydrophilicity of the hydrogels was evaluated by measuring the water contact angle using sessile drop technique. A high-speed camera was used to capture the momentary droplet images when the water droplet (2 μL) was placed on the surfaces. Subsequently, the contact angles were calculated using a well-known method.
2.6. Swelling Test
To prepare the samples for swelling test, the cylindrical module was provided in our lab, 1 mL solution of alginate 2% (w/v) and 3% (w/v) was mixed via 100 µL CaCl2 200 mM containing 0.1 % (w/v) chitosan nanoparticles. The solution was formed within few seconds. The hydrogel specimen was placed in a glass tube that contained 1:10 (v/v) KRH buffer for reaching equilibrium state of swelling at ambient temperature. The hydrogel was gently removed from KRH solution and remove excess liquid by blotting with paper tissue. The wet weight of hydrogel was weighted. The swelling ratio of hydrogel was determined from dividing the weight of hydrogel at indicated time points soaking in KRH and initial weight of the hydrogel.
2.7. Degradation Study
The degradation behavior of hydrogel was studied by incubating totally swelled 2% (w/v) and 3% (w/v) alginate hydrogel containing 0.1% (w/v) chitosan nanoparticle (5 mm× 5 mm × 4 mm dimensions) in 10 mL PBS containing alginate lyase 0.5 unit/mL (pH 7.4) and culture media containing 0.1% (w/v) sodium azide to prevent microbacterial growth at 37°C. The hydrogel was taken out and weighted at pre-defined time intervals. The degradation was characterized by hydrogel fraction based on the initial weight of the totally swelled hydrogel.
2.8. Cell Viability and Proliferation Assay
Cell viability was measured by colorimetric assay using the tetrazolium salt MTT (3-[4, 5-dimethylthiazol-2-y1]-2, 5-diphenyltetrazolium bromide) reduction to formazan crystal products. The EnScs at 2 × 105 cells/well were seeded in hydrogel without and with taurine loaded chitosan nanoparticles in 24-well tissue culture plates and incubated for 84h. The hydrogel was gently rinsed with PBS (pH 7.4) at indicated time points and MTT solution (5 mg/mL) was added into each sample and incubated at physiological temperature for 4h. After removal of media, dimethylsulfoxide was added to samples and the absorbance automatically was measured with a microplate spectrophotometer at 570 nm.
3. Results and Discussion
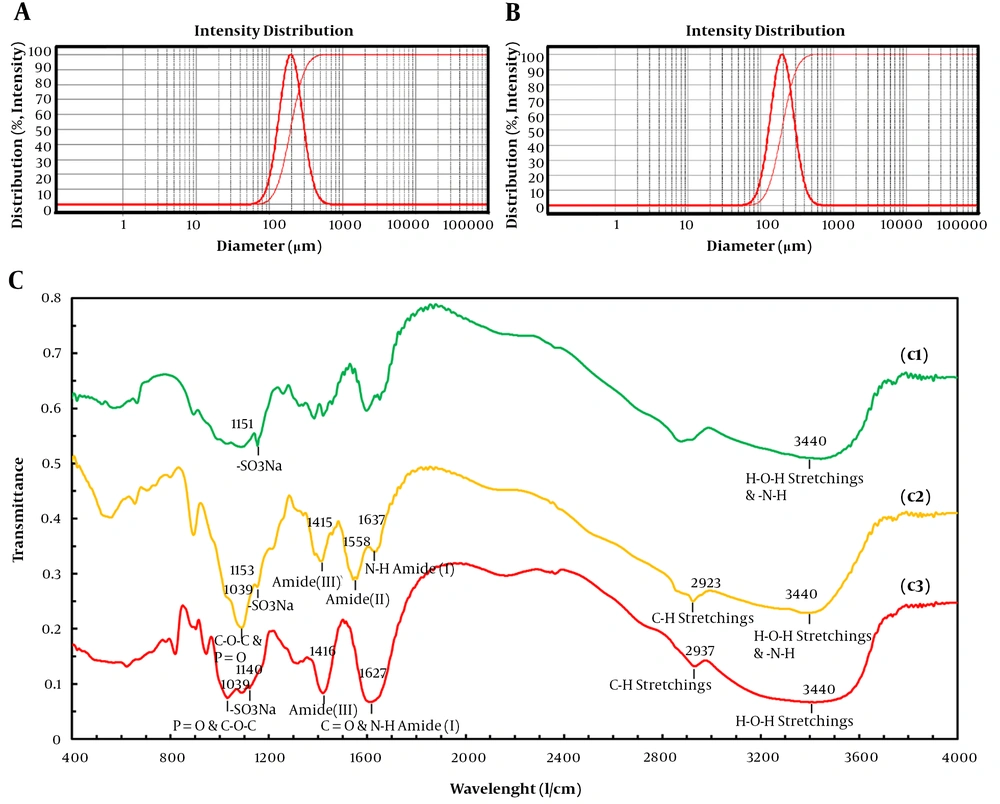
The surface charge of hydrogel scaffold was evaluated by measuring zeta potential of the substrates, which indicated formula stability. The chitosan nanoparticle was positively charged (value + 53.5 mV), which was in accordance with the cationic nature of chitosan (neat chitosan value +46.6 mV) and results confirmed the successful synthesis of the nanoparticle. The chitosan nanoparticle showed a higher value of charged intensity compared with neat chitosan, which could be due to the increase of charged molecules on the surface of the nanoparticle. Through ultrasonication preparation chitosan nanoparticle, 1,4-glycosidic bonds were broke and dissociated chitosan aggregates formed due to the Brownian motion of the particles and adhesive nature of chitosan causes their attachment (16-18). Moreover, incorporation of taurine into chitosan nanoparticle decreased in zeta potential value +41.1 mV and smaller positive zeta potential due to negative charge property of taurine. However, chitosan nanoparticles containing taurine encapsulated in alginate-based hydrogel did not experience a significant decrease in its zeta potential value +35.3 mV. The positive zeta potential of hydrogel substrates contributes to the suitable adherence of scaffold to negatively charged biological molecules. Nanoparticles formulated with an average diameter of 210 and 187 nm, respectively for both of the taurine free and loaded samples (Figure 2A and B). The taurine free and loaded chitosan nanoparticles showed narrow size distribution having a low polydispersity index of 0.214 and 0.238, respectively. The presence of taurine and its loading in nanoparticles had a small influence in size, by decreasing about 20 nanometers. This could be attributed to the electrostatic interaction and ionic crosslinking between negatively charged taurine and positively charged chitosan molecules, which led to electrostatic force of attraction among substrates and finally particle shrinkage. The narrow polydispersity was revealed about nanoparticle homogeneity in the prepared formulation (Figure 2A). The LF% and EF% of taurine in nanoparticles was 20% and 68%, respectively, which proved a high degree of encapsulation through the process. This could be due to the suitable concentration of crosslinking reactants, including TPP and electrostatic interactions between oppositely charged groups, or dispersion forces (12, 18). The ability of the crosslinked structure of anionic taurine with cationic chitosan to sustain the drug release and cytocompatibility of synthesized hydrogels are exploited in this method during an extended time of incubation.
Dynamic light scattering (DLS) of synthesized taurine-free chitosan nanoparticle (A) and taurine-loaded chitosan nanoparticle (B) and FTIR spectrum of substrates in different compositions (C). Neat taurine (c1) taurine-loaded chitosan nanoparticle (c2), and alginate hydrogel containing taurine-loaded chitosan nanoparticle (c1).
3.1. FTIR Analysis
FTIR spectra of scaffold substrates are shown in Figure 2C. The FTIR spectrum of taurine-loaded chitosan and alginate-containing taurine-loaded nanoparticles showed a broad -OH stretching and amide (-N-H) band between 3600 and 3150 cm-1. The intensity of the amide peak for chitosan corresponds to the partial N-deacetylation of chitin. The peak for N-H stretching vibration of amine I at 1637 cm-1, amide II carbonyl stretch at 1558 cm-1, amide III at 1414 cm-1, respectively. The broadband from 1540 to 1647 cm-1 that arose from overlapping bands from amide of chitosan and carboxyl anions of alginate confirmed the electrostatic interaction between alginate and chitosan. The P = O was attributed to the linkage between phosphoric and ammonium ion in which the tripolyphosphoric groups of TPP were linked with ammonium groups of chitosan and C-O-C stretching of chitosan and alginate was shown at 1039 cm-1. The peaks for asymmetric and symmetric stretching vibration bands of sulfonate (O = S = O) group were shown at 1130 and 1200 cm-1. These results indicated that taurine and alginate had been grafted onto the chain of chitosan without breaking the structure.
3.2. Contact Angle Measurement
The contact angles of all samples were assessed by Zisman method. Among natural polymers, alginate has been extensively used in biomedical application because of its superior degradation ability and nontoxicity of its product. However, rich carboxylic acid (-COOH) and hydroxyl (-OH) groups on this polymer limit cell adhesion (19, 20). Chitosan grafting is a common way to enhance hydrophobicity (19, 20). In this study, the existence of nanoparticles clearly decreased the contact angle from 97 ± 3° in alginate hydrogel to 61 ± 4° in alginate/chitosan nanoparticle hydrogel. Compositing the alginate with chitosan nanoparticles decreases its hydrophilicity because chitosan is low hydrophilic rather than alginate. Furthermore, no significant difference was observed between taurine free and loaded chitosan nanoparticle/alginate hydrogels, which proved the existence of chitosan nanoparticles individually could promote hydrophobicity of alginate-based hydrogels.
3.3. Swelling Test
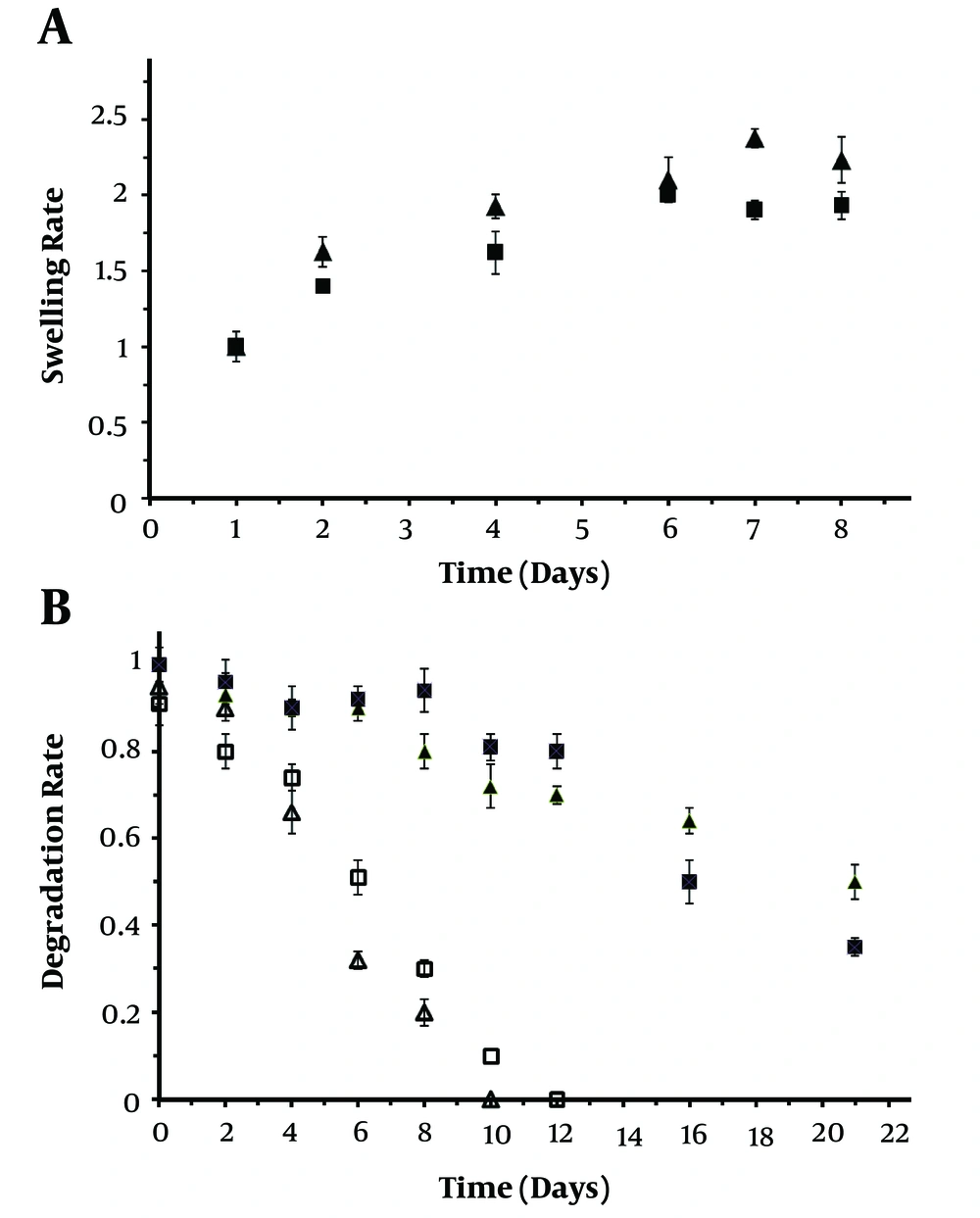
The polymer networks absorb water molecules, resulting in the swelling of the hydrogels. The swelling ratio can be used as a simple method to characterize stability and water uptake of hydrogels. Permeation of nutrients or air and also cellular metabolic products into hydrogels and out of hydrogels, respectively will be evaluated with the degree of swelling (19-21). The ratio of swelling hydrogels in a time-dependent response is shown in Figure 3A. It was explored that hydrogels attained equilibrium swelling until 8 days. The alginate hydrogel containing taurine-loaded chitosan nanoparticle showed a higher water uptake compared with neat alginate hydrogel that could be due to the smaller pore area in the hydrogel network, which retained much water (bulk liquid). In details, it is assumed that water bulk fills the area between the network chains, most center of larger pores, and macro-pores or voids. The hydrogels containing taurine-loaded chitosan nanoparticle had 30% more swelling rate than alginate scaffold until 8 days of incubation at the physiological condition. The alginate-containing taurine-loaded chitosan nanoparticle hydrogel exhibits a higher swelling sensitivity compared with alginate hydrogels, which extends their potential application. The results proved that the surface hydrophilicity of the taurine-loaded chitosan nanoparticle/alginate hydrogel significantly was increased compared with neat alginate hydrogel (6, 10, 11).
3.4. In Vitro Degradation of Hydrogel
Degradation of hydrogel is recognized as another crucial specification that manages the stability of the scaffold under physiological conditions (10, 20, 21). As blood serum is composed of different inorganic ions such as sodium, potassium, calcium, and magnesium, as well as organic compounds such as uric acid and amino acids, the evaluation of in vitro degradation of fabricated scaffold in a media containing different salts has a significant role in its biomedical application. Therefore, in this research, DMEM containing 10 % FBS was chosen in order to assess the degradation rate of fabricated hydrogel (Figure 3B). The scaffolds were gradually degraded after incubation in the PBS buffer until 21 days. Meanwhile, the degradation of taurine-loaded nanoparticle/alginate hydrogel was slower than neat alginate hydrogel until 2 and 12 days soaking in alginate lyase and PBS solution, respectively. The degradation rate of taurine-loaded chitosan nanoparticle/alginate hydrogel was significantly faster than neat alginate hydrogel during an extended time of incubation (Figure 3B). Moreover, both of the neat alginate and taurine loaded nanoparticle/alginate hydrogel were degraded in higher speed after initial days of incubation. The incorporation of taurine-loaded chitosan nanoparticle/alginate hydrogel prolonged hydrogel degradation through enzymatic treatment until 12 days of incubation. Also, taurine-loaded chitosan nanoparticle/alginate hydrogel compared with neat alginate hydrogel showed higher stability and lower degradation rate in PBS solution. Formation of polysaccharide complexes between the oppositely charged chitosan and alginate by attractive electrostatic interactions can be the reason for obtained results (6, 13, 14).
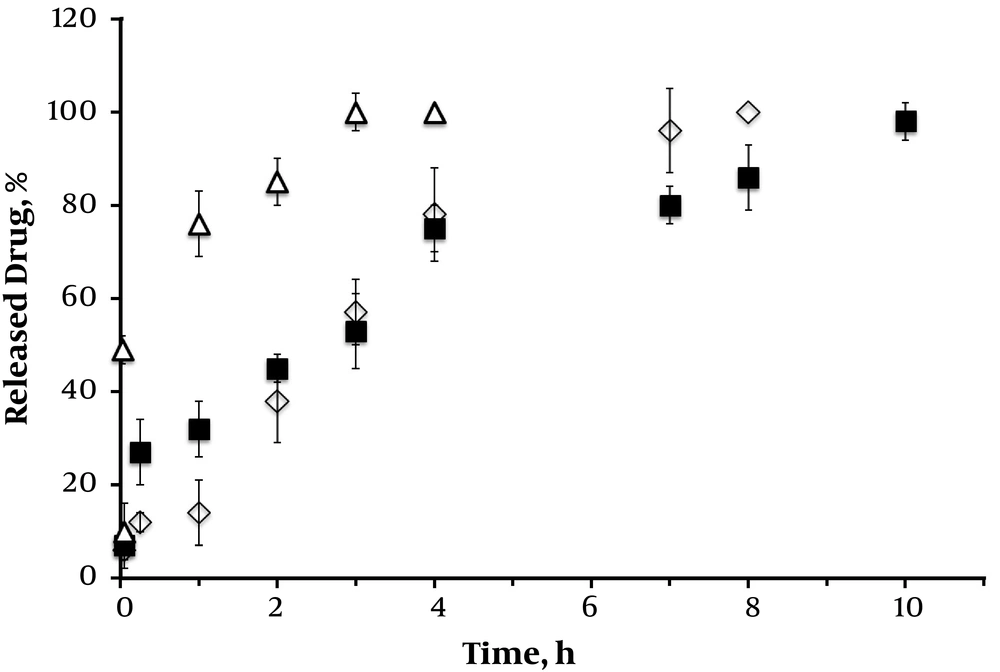
3.5. Taurine Release
To determine in vitro drug release of taurine from samples, including taurine-loaded nanoparticle and with alginate hydrogel, the cumulative release was measured at a certain time of incubation. Figure 4 shows the cumulative percentage of drug release from nanoparticles initially burst release and sustained release during the extended time of incubation. The sustained release is attributed to the presence of protective alginate hydrogel that operates as a rate-limiting membrane, which controls the release of the taurine molecules. The taurine-loaded chitosan nanoparticle formulation had a rapid release of about 75% within 24 h, indicating a higher speed in initial hours. The sustained slow release was obtained until 84 h of incubation. As seen in Figure 4, there was more moderate taurine release pattern in the alginate-based scaffold formulations more than 190 h due to their entrapment efficiency. This could be explained by the differences in ionic charge of substrates used in the preparation of hydrogel (11, 14, 17, 18). The release of taurine depended on the alginate concentration as the drug release was hindered with the incorporation of alginate in higher concentrations as a consequence of viscosity and intensity of alginate scaffold. The sustained release of taurine could minimize the rate of reapplication (14, 17, 18).
3.6. SEM Analysis of Scaffolds
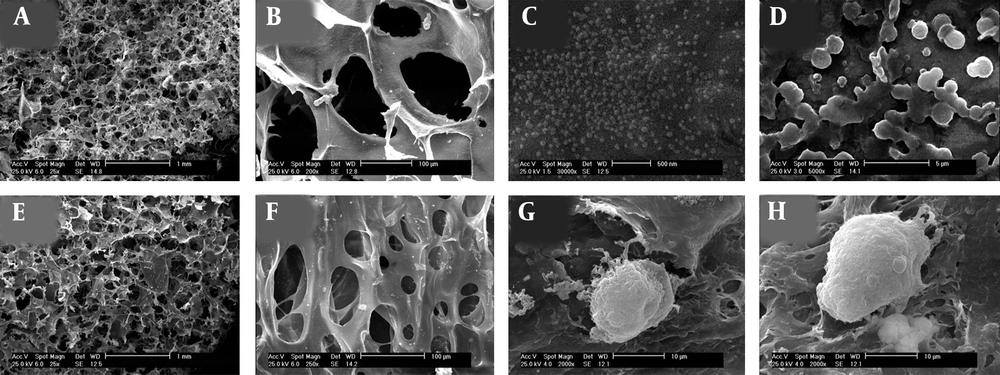
Structure and architecture of the fabricated scaffolds and cell attachments were observed by SEM analysis. The porosity of hydrogels obtained from different cross sections of the prepared scaffolds by the measurement of averages of Image J software values and SEM images. The morphology of both neat alginate and taurine-loaded chitosan nanoparticle/alginate hydrogels are shown in Figure 5. The appearance of chitosan nanoparticle incorporated in alginate hydrogel confirmed the homogenous distribution of chitosan nanoparticle throughout the hydrogel structure. The pores were interconnected and had almost spherical geometries ranging from 30 to 150 µm in size. This interconnected porous-like appearance could promote cell attachment, the proliferation as well as migration in tissue engineering. The mean porosity of the hydrogels was calculated to be 83 ± 4%. Addition of 0.1% chitosan nanoparticles to hydrogel did not affect pore shape, size and the percentage of scaffold porosity (Figure 5B and F). A high porosity with an open pore structure and a high degree of interconnectivity are seen in Figure 5. Furthermore, the porous structure of fabricated scaffolds could improve the delivery of oxygen, nutrients, and metabolites throughout the system. SEM images show the morphology of cells cultured for 84h on fabricated hydrogels (Figure 5G and H). Cells attached to the surface of hydrogels and exhibited a well spheroid morphology, which could be explained by the lack of cell adhesion moieties in prepared hydrogels. The cells were entrapped on hydrogel scaffolds physically rather than biochemically (14, 17, 18).
SEM image of composite hydrogels (A, B) alginate hydrogel in different magnification, (C, D) taurine-loaded chitosan nanoparticle, (E, F) alginate hydrogel containing taurine-loaded chitosan nanoparticles in different magnification, and (G, H) cells were encapsulated in taurine-free and loaded chitosan nanoparticle/alginate hydrogels, respectively.
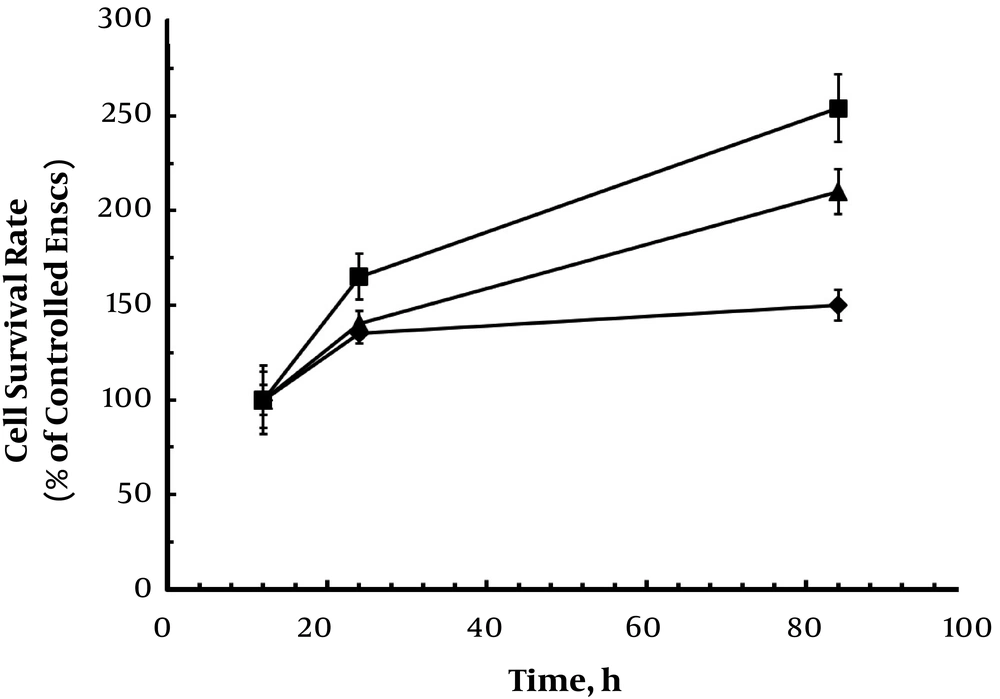
3.7. EnSCs Viability
EnSCs encapsulation in hydrogels on cell viability was measured by MTT assay after 84h. The survival rate of encapsulated cells was comparable with conventional cultured EnSCs on PS at 12 h (Figure 6). The encapsulation of cells in the hydrogels free and taurine-loaded chitosan nanoparticle did not decrease the viability of cells. Monitoring the cell viabilities displays a cytocompatibility of hydrogels as cell number increased with time (Figure 6). Also, the results indicated cell growth in the hydrogels during the extension time of culture, which could be due to high pore size and proper porosity of produced hydrogels. In addition, incorporation of chitosan nanoparticle showed a higher rate of cell survival, which would be due to cell adhesion property of chitosan. However, the conventionally cultured cells on PS grew faster than encapsulated cells until 84h. The results indicated that the cell growth suppression arising from microscopic stress by surrounding environment could particularly suppress cell proliferation (6, 11-14).
4. Conclusions
The calcium-alginate hydrogel with chitosan nanoparticle prepared in this study could be a suitable polymeric carrier for the taurine drug as a bioactive substrate in tissue engineering and drug delivery systems. In addition, chitosan nanoparticle enhanced release time of taurine within hydrogel due to electrostatic interaction among positively- and negatively charged substances through positively charged chitosan and negatively charged alginate and taurine. Blending of chitosan nanoparticles with alginate has been used to adjust the swelling and degradability of hydrogels. In summary, the composite alginate-based hydrogel containing taurine-loaded chitosan nanoparticle due to degradability and biocompatibility showed suitable microenvironment for tissue engineering and controlled drug released systems.