1. Background
One of the most complications after intra-cranial surgery is meningitis. Post-operative meningitis (POM) is an uncommon (0.3 - 8.1%) but life-threatening complication with high morbidity and sometimes mortality reported up to 50% (1-5). Also, postoperative meningitis is associated with prolonged hospitalization, multiple surgeries, long time antibiotic treatment and increase in total cost of illness (6, 7). It is important to identify risk factors of POM to decline rate of mortality Multiple risk factors have been known, such as postoperative cerebrospinal fluid leakage, concurrent incision infection, CSF shunts, long time operation over 4 hours and emergency surgery (8, 9). As well as set of a foreign body duration surgery, an absence of antibiotic prophylaxis, previous radiation therapy, previous neurosurgical infection and interventions on the nasal sinuses have been reported as related factors to POM (8-10). A strong clinical suspicion for meningitis diagnosis is in patients who have the triad of fever, neck stiffness and mental status alteration during the early post-operative period (11, 12) but these signs are nonspecific for diagnosis of post-operative meningitis (13). Analysis of CSF can be helpful for diagnosis of disease but post-operative CSF markers including, glucose, protein, lactate, and cellularity also have variation because of manipulating the brain, background brain pathology or bleeding into CSF following the surgery (4, 14). According to the study by Kourbeti et al. in 2015, the incidence rate of post neurosurgery meningitis has been reported to be about 4.8% and CSF cultures were positive for microbial growth in 100% of these cases. Steroid use, CSF leakage, and ventricular drainage were risk factors to POM mortality (6). In a study by van Aken et al. MO in 2004, post-operative meningitis occurred in 0.7% of patients and all patients underwent the external lumbar drainage (ELD) immediately after surgery for at least 5 days (15).
2. Objectives
Although there are many known risk factors for POM incidence, research on related factor to mortality is limited. Thus this study was aimed to investigate on the factors associated with the mortality of patients diagnosed with post-operative meningitis referred to Loghman Hakim hospital as a tertiary university hospital over 2017 - 2018.
3. Methods
In this descriptive follow-up study conducted among 425 patients who had undergone neurosurgery (open or closed) in Loghman Hakim Hospital from March 2017 to March 2018, 34 (8.9%) patients were diagnosed with post-operative meningitis. All patients received appropriate prophylactic antibiotics, at the induction of anesthesia. For diagnosis of post-operative meningitis, we used criteria of centers for disease control which are as follows:
1) Organisms found in CSF culture
2) Signs or symptoms with no other recognized cause (at least one): Fever more than 38°, headache, neck stiffness, meningeal, cranial nerve signs, or irritability and presence of at least one of the following:
a) Elevated WBC count and protein level, and or declined CSF glucose level
b) Organisms found in gram stain of CSF
c) Organisms found in blood culture
d) Affirmative antigen test for CSF, blood, or urine
e) Diagnostic titer (IgM) or 4-fold elevation (IgG) for pathogen (16-18).
After being ensured of clinical suspicion of POM, all patients received empiric antibiotic regimen. Data of patients with high suspicion of POM include, demographic characteristics (age, sex), clinical manifestation (headache, fever, consciousness Alternation), cause of neurosurgery, elective or emergency surgery, having mechanical ventilation, surgical site infection (SSI), duration of surgery, length of hospital stay before surgery, having CSF shunt or ventricular drain, reoperation, as well as total length of ICU and hospital stay, use of corticoid, use of antibiotic prophylaxis, also, CSF analysis/lactate and culture results collected in a data collecting form designed by researchers. All the patients were followed and outcome of patients was estimated during 30 days after diagnosis. Informed consent was obtained from patients or first degree relatives before neurosurgery to participate in this study. This study was approved by the Ethics Committee of Shahid Beheshti University of Medical Sciences, Tehran, Iran.
3.1. Statistical Analysis
For the report of descriptive results, the mean, standard deviation (SD) as well as number and percentage were used. For data analysis, the chi-squared and Fisher exact test as well as independent t-test and Mann-Whitney U tests were used. In order to predict possible factors related to the outcome of patients with post-operative meningitis, multivariable logistic regression analysis was performed. Also, the survival curve was plotted. Total analysis was performed using SPSS software version 19. A significant level was considered P < 0.05 for all tests.
4. Results
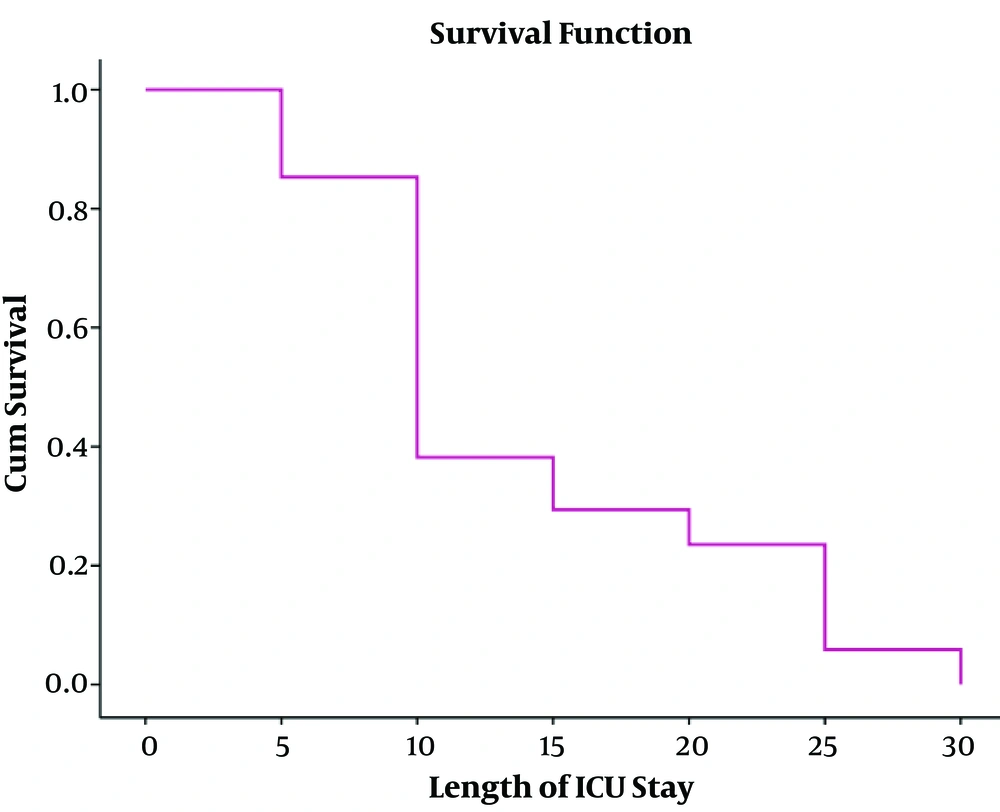
In this study, the mean age ± SD of 34 (8.9%) patients diagnosed with post-op meningitis was equal to 46.41 ± 16.94 years’ old which 52.5% of them were male. The most common causes of surgery included a brain tumor (44.1%), the pituitary adenoma, hydrocephalus, cerebrovascular diseases (subarachnoid hemorrhage, aneurysm, intracranial hemorrhage, and malformations), trauma, and colloid cysts, respectively. The mean length of hospital stay before surgery was equal to 2.26 ± 2.95 days. The mortality rate of post-operative meningitis was equal to 50%. Total characteristics of patients with post-operative meningitis have shown in Tables 1 and 2. In bivariable analysis, there was no association between outcome (death and alive with or without disability) of patients affected by post-operative meningitis and age (P = 0.08), reoperation (P = 0.5), ventricular drain or shunt (P = 0.9), length of hospital stay before surgery (P = 0.3), duration of surgery (P = 0.2), length of ICU stay (P = 0.05) as well as length of hospital stay (P = 0.06), CSF lactate (P = 0.07), CSF leakage (P = 0.6), positive CSF culture (P = 0.1), CSF analysis (leukocyte count P = 0.7, glucose P = 0.8, protein P = 0.9),surgical site infection (P = 0.5). Only there was a statistically significant association between mortality of POM and male gender (P = 0.01) and having mechanical ventilation (P = 0.002) (Table 3). It means that 64.7% and 94.1% of death happened in male gender and patients who needed mechanical ventilation, respectively. In multivariable logistic regression analysis, length of ICU stay more than 7 days (P = 0.04, OR = 1.2, 95% CI = 1.02 - 6.2), having mechanical ventilation (P = 0.03, OR = 1.1, 95% CI = 1.05 - 5.1), positive CSF culture (P = 0.01, OR = 2.4, 95% CI = 1.9 - 4.08) were predictor factors for mortality (Table 4) (23). In this study, we found an inverse relationship between patient’s survival and length of ICU stay (Figure 1) (23).
| Variables | Valuesa |
|---|---|
| Age, y | 46.41 ± 16.94 |
| Sex | |
| Male | 18 (52.5) |
| Female | 16 (47.1) |
| Diagnosis | |
| Brain tumor | 15 (44.1) |
| Pituitary adenoma | 5 (14.7) |
| Hydrocephalus | 7 (20.6) |
| Cerebrovascular disease | 4 (11.8) |
| Trauma | 2 (5.9) |
| Colloid cyst | 1 (2.9) |
| Length of hospital stay before surgery, d | 2.26 ± 2.95 |
| Duration of surgery, h | 5.49 ± 2.56 |
| The time between surgery and POM diagnosis, d | 3.67 ± 2.95 |
| Type of surgery | |
| Open | 32 (94.1) |
| Close | 2 (5.9) |
| Surgery status | |
| Emergency | 11 (32.4) |
| Elective | 23 (67.6) |
| Mechanical ventilation, d | 10.04 ± 10.76 |
| Yes | 23 (67.6) |
| No | 11 (32.4) |
| Use of corticosteroid | |
| Yes | 34 (100) |
| No | 0 (0) |
| Prophylactic antibiotics | |
| Yes | 34 (100) |
| No | 0 (0) |
| Post-operative ventricular drain or shunt | |
| Yes | 8 (23.5) |
| No | 26 (76.5) |
Basic Characteristics of Patients with Post-Operative Meningitis
| Variables | Valuesa |
|---|---|
| Headache | |
| Yes | 23 (65.7) |
| No | 12 (34.3) |
| Fever | |
| Yes | 18 (52.5) |
| No | 16 (47.1) |
| Consciousness alternation | 9.85 ± 3.38 |
| Yes | 25 (73.52) |
| No | 9 (26.5) |
| CSF culture | |
| Yes | 5 (14.7) |
| Acinetobacter | 4 (11.8) |
| Staph | 1 (2.9) |
| No | 29 (85.3) |
| CSF lactate | 55.57 ± 32.68 |
| CSF analysis | |
| CSF leukocyte count, mm3 | 486.24 ± 424.22 |
| CSF glucose, mg/dL | 40.35 ± 9.80 |
| CSF protein, mg/dL | 143 ± 74.47 |
| Outcome | |
| Death | 17 (50) |
| Mild disability | 2 (5.9) |
| Recovery | 15 (44.1) |
| Length of ICU stay | 11.05 ± 7.66 |
| Length of hospital stay | 24.44 ± 15.44 |
Clinical and Paraclinical Findings of Patients with Post-Operative Meningitis
| Variables | Death | Alive | P Value |
|---|---|---|---|
| Age | 51.47 ± 17.92 | 41.35 ± 14.70 | 0.08 |
| Sex | 0.01c | ||
| Male | 64.7 | 41.2 | |
| Female | 35.3 | 58.8 | |
| Surgery status | 0.2 | ||
| Elective | 76.5 | 58.8 | |
| Emergency | 23.5 | 41.2 | |
| Mechanical ventilation | 0.002c | ||
| Yes | 94.1 | 41.2 | |
| No | 5.9 | 58.8 | |
| Reoperation | 0.5 | ||
| Yes | 5.9 | 11.8 | |
| No | 94.1 | 88.2 | |
| Post-operative ventricular drain or shunt | 0.9 | ||
| Yes | 23.5 | 23.5 | |
| No | 76.5 | 76.5 | |
| Length of hospital stay before surgery, d | 0.3 | ||
| 7 ≥ | 100 | 88.2 | |
| 7< | 0 | 11.8 | |
| Duration of surgery, h | 0.2 | ||
| 4 ≥ | 50 | 29.4 | |
| 4 < | 50 | 70.6 | |
| Length of ICU stay, d | 13.82 ± 7.93 | 8.29 ± 6.47 | 0.05 |
| Length of hospital stay, d | 23.70 ± 17.78 | 27.17 ± 13 | 0.06 |
| CSF lactate | 59.72 ± 43.88 | 51.42 ± 15.55 | 0.07 |
| CSF leakage | 0.6 | ||
| Yes | 5.9 | 17.6 | |
| No | 94.1 | 82.4 | |
| Positive CSF culture | 0.1 | ||
| Yes | 23.5 | 5.9 | |
| No | 76.5 | 94.1 | |
| CSF analysis | |||
| CSF leukocyte count, mm3 | 508 ± 410.53 | 463.22 ± 48.95 | 0.7 |
| CSF glucose, mg/dL | 40.68 ± 9.53 | 40 ± 10.3 | 0.8 |
| CSF protein, g/dL | 140.63 ± 76.41 | 145.61 ± 74.48 | 0.8 |
| Surgical site infection | 0.5 | ||
| Yes | 23.5 | 17.6 | |
| No | 76.5 | 82.4 |
Relationship Between Outcomes of Patients and Studied Variables in Post-Operative Meningitisa
| Variables | Surveyed | Reference | P Valuea | OR (95% CI) |
|---|---|---|---|---|
| Length of ICU stay, d | < 7 | ≤ 7 | 0.04 | 1.02 (1.01 - 6.2) |
| Mechanical ventilation | Yes | No | 0.03 | 1.1 (1.05 - 5.1) |
| Positive CSF culture | Yes | No | 0.01 | 2.4 (1.9 - 4.08) |
Positive Results of Multiple Logistic Regression Analysis to Predict Mortality Outcome in Patients Affected by Post-Operative Meningitis
5. Discussion
In this survey, the incidence of post-operative meningitis was equal to 8.9%. Only 14.7% of patients had positive CSF cultures. Two organisms found were gram-negative bacilli (Acinetobacter) (11.8%) followed by gram-positive cocci (Staphylococcus aureus) (2.9%). This rate varies from one study to another. Kourbeti et al. in a study conducted in 2007 indicated that the incidence of post-operative meningitis was equal to 5% that the most common organisms found were gram-positive cocci (7). Dashti et al. indicated that the most common organisms causing meningitis were gram-negative with priority Bacillus, Pseudomonas and Klebsiella species (19). In one of the largest neurosurgery studies, the incidence of meningitis after neurosurgery procedures was < 1% (20). Low incidence of POM (< 1%) may be related to the prescription of antibiotics prophylaxis. But, in the present study, despite receiving the antibiotic prophylaxis in all patients, the incidence rate of post-operative meningitis was reported by 8.9%. Another study indicated that antibiotic prophylaxis reduced incision infections from 8.8%to 4.6% (P < 0.0001) but did not prevent meningitis: 1.63% in patients who did not receive antibiotic prophylaxis, and 1.50% in those who received prophylaxis antibiotic (10). In the current study, 52.5% of patients affected by post-operative meningitis were men. There was a statistically significant association between male gender and death outcome in chi-squared analysis (P = 0.01). Kono et al. in a paper published in 2011 indicated that male gender (P = 0.02, OR = 3.97, 95% CI: 1.21 - 13.03) was a risk factor causing post-operative meningitis (21). The mean length of hospital stay before surgery (days) and duration of surgical procedure (hours) were equal to 2.26 ± 2.95 and3.67 ± 2.95 respectively. In a study by Rezaei et al. long time operation over 4 hours and length of hospital stay before surgery more than 7 days were predictor factors for onset of post-operative meningitis (8, 9). In this study, there was no statistically significant association between mortality and the majority of variables studied such as length of hospital stay before surgery (over 7 days), duration of surgery (over 4 hours), surgical status (elective, emergency) (P = 0.2), reoperation (P = 0.5), CSF leakage (P = 0.6), post-operative ventricular drain or shunt (P = 0.9), length of ICU-hospital stay (P = 0.06), CSF lactate (P = 0.07), SSI (P = 0.5), CSF analysis (leukocyte, glucose and protein) (P = 0.7, P = 0.8, P = 0.8). In the published study by Erdem et al. in 2008, the mortality rate of POM reported by 40.8% which in the logistic regression analysis model, Glasgow coma scale (GCS) score less than 10 (OR: 19.4, 95% CI: 1.6 - 23, P = 0.001), and low CSF glucose level (≤ 30 mg/ dL) (OR: 10.2, 95% CI; 1.2 - 82.8, P = 0.002), and presence of concurrent nosocomial infection (OR: 28.744, 95% CI; 1.6 - 501, P = 0.001) were the nondependent risk factors associated with mortality (22). According to our results, there was a statistically significant association between having mechanical ventilation and mortality of post-operative meningitis (P = 0.002) with 94.1% of death outcome in patients affected by POM who needed mechanical ventilation. In addition, length of ICU stay (P = 0.04, OR = 1.2, 95% CI = 1.02 - 6.2), mechanical ventilation (P = 0.03, OR = 1.1, 95% CI = 1.05 - 5.1), positive CSF culture (P = 0.01, OR = 2.4, 95% CI = 1.9 - 4.08) were predictor factors for death outcome in multiple logistic regression analysis. In a study by Kourbeti et al. the risk of meningitis was related to pre-operative steroid use (P = 0.005, OR = 11.55), CSF leakage (P < 0.001, OR = 48.03) and ventricular drainage (P < 0.001, OR = 70.52) (6). Other study showed that the cerebrospinal fluid leakage, diabetes mellitus, and male gender were not associated with an increased incidence of postcranial neurosurgery infection (20). Positive results found only in CSF culture related to 5 patients may be due to the prescription of pre-operative prophylaxis antibiotics during neurosurgery. As mentioned in current and other studies many environmental factors can influence the incidence and outcome of patients with meningitis after neurosurgery (23). The limitation of this study was a low number of post-operative meningitis cases which could reduce the power of statistical tests and as a result leads to a decrease in the generalizability of results. It is recommended to conduct multicenter studies with more sample size in a long time.