1. Background
Aphasia is an acquired language disorder that is caused by damage to the language-dominant (mostly left) hemisphere regions (1). This disorder is characterized by poor receptive and expressive language skills in both oral and written language (2). However, lots of studies have indicated that non-language cognitive impairments, including executive function (EF) deficits, usually coincide with aphasia and, notably, may influence language profiles and outcomes (3). executive function (also called executive control or cognitive control) is an umbrella term that includes a set of top-down mental processes with three core components: (a) inhibition or inhibitory control, including self-control and interfering control, (b) working memory (WM), and (c) cognitive flexibility (also called set-shifting, mental flexibility, or mental set-shifting) (4). As mentioned, set-shifting reflects cognitive flexibility: Being able to alter one’s own perspective spatially or interpersonally. To alter perspectives, the deactivation of our former perspective and loading of a different perspective to WM is required. In this regard, set-shifting builds on inhibitory control and WM (5).
Set-shifting was first described by Jersild (1927) and is measured through task switching paradigm (6). The oldest of these tasks is likely the Wisconsin Card Sorting Task (WCST); one of the classic tests of prefrontal cortex functions. Each card can be sorted by color, shape, or number. The subject should infer the correct sorting criterion based on the feedback provided by the examiner. Whenever the examiner gives feedback about the change of the sorting principle, she/he ought to alter her sorting technique flexibly. The test requires minimum instructions and does not call for verbal responses from the examinee. This makes it suitable for PWA.
Previous investigations have demonstrated PWA ability to confront problems involving executive control tasks. Baldo et al. (7) revealed that problem-solving skills were significantly correlated with the degree of language deficiency in PWA. Murray (8) demonstrated that many, yet not all, PWA in their study showed EF deficits. Moreover, the data from correlations among EF and language measurements revealed domain-general cognitive problems in PWA. In this regard, Kuzmina and Weekes (9) found certain links between cognitive deficits of PWA and their language comprehension and production abilities.
Some investigators believe that executive functioning and language are mutually related, that is, each skill of one part is dependent on or is related to certain skills on the other side (5). Other researchers have even suggested that a number of language elements, such as comprehension, are robust predictors of EF abilities (10). Also, a class of linguistic tasks comprised of verbal and semantic fluency tests exploit cognitive flexibility (4). Language production and comprehension correlate with major cognitive components (11, 12). Attention, perception, memory, set-shifting and language are inter-related cognitive functions of the brain. Although memory in PWA has received much attention throughout the literature, EF needs further exploration in terms of aphasic language deficits (3). This is also true for set-shifting. The executive function of switching between tasks or set-shifting occurs unconsciously, in which attention automatically swings between different tasks. If language and cognition are considered as a whole system, a relationship between set-shifting and language skills/deficits may be examined in PWA (13). In other words, the question is: What happens to WCST responses (and set-shifting status) when language is disrupted in PWA. Furthermore, to the best of the authors’ knowledge, there has been no study regarding set-shifting ability in Persian-speaking PWA to date.
2. Objectives
The purposes of this study was two-folds: (1) To determine the relationship between set-shifting and language skills/deficits in PWA; and (2) To investigate, which aspects of language (e.g., naming, fluency, and comprehension) are most related to set-shifting in Persian-speaking PWA.
3. Methods
3.1. Participants
Nineteen non-fluent PWA (13 males and six females; with mean age of 54.26 ± 8.88 years and age range of 32 to 69 years) participated in this study. They were selected based on their availability and willingness to participate in Tehran (Iran) between November, 2016 and August, 2017. The inclusion and exclusion criteria are shown in Box 1. A neurologist identified the location of lesions using clinical scans of magnetic resonance imaging (MRI) and computed tomography scan (CT). Prior to the study, participants signed a consent form regarding the study. The study received approval from the ethics committee of the University of Social Welfare and Rehabilitation Sciences under the ethics code IR.USWR.REC.1395.75.
| Criteria |
|---|
| Inclusion criteria |
| Aphasia after stroke |
| Time post-onset ≥ 6 months |
| Age 18 - 70 years |
| Native speaker of Persian |
| Right-handed (based on the Edinburgh handedness inventory) (14) |
| Exclusion criteria |
| Global aphasia, defined as P-WAB-1 score < 25 |
| Severe speech apraxia, defined by verbal apraxia tasks for adults (15) |
| A history of psychiatric disease |
| Presence of any signs of dementia or neurocognitive disorder |
Inclusion and Exclusion Criteria
3.2. Procedures
This study was a cross-sectional, observational study designed to assess set-shifting skills and their correlation with language skills/deficits of Persian-speaking PWA. Patients were administered with the manual version of the WCST and the bedside version of the Persian Western Aphasia Battery (P-WAB-1) in separate testing sessions over two consecutive days. These tests were administered in a quiet and private place. In order to explain how to perform the tests to the PWA, the same guidelines were used as for normal WCST subjects (16).
3.3. Materials
3.3.1. Bedsides the Version of P-WAB-1
To assess speech and language functions, the bedside version of the Persian Western Aphasia Battery (P-WAB-1) was used. This test consists of six language subtests as follows: (1) spontaneous speech content; (2) fluency of spontaneous speech (fluency); (3) auditory comprehension involving ten Yes/No questions; (4) sequential commands covering five commands of different complexities; (5) repetition involving six words and sentences of different lengths; and (6) naming containing 20 different naming categories. Each subtest of P-WAB-1 obtained a raw score of 10. As suggested in the manual of Western Aphasia Battery-Revised (WAB-R), a percentile Aphasia Quotient (AQ) can be calculated based on the raw scores to determine the severity of aphasia (17). Based on the AQ range of the P-WAB-1, AQ score of 0 to 25 is categorized as very severe aphasia, an AQ of 26 to 50 as severe aphasia, an AQ of 51 to 75 as moderate aphasia, and an AQ of 76 to 92 as mild aphasia. The internal consistency (a = 0.71) and test-retest reliability (r = 0.65, P < 0.001) of the P-WAB-1 were satisfactory. The subtests are sensitive enough to contribute to AQ as a functional measure of severity of aphasia in brain-damaged patients (18).
3.3.2. The WCST
In the current study, the WCST-64 Card Version (19) was used as a measurement of EF. Upon administration of the WCST-64, subjects were asked to sort 64 response cards by color, shape, or number, based on four stimulus cards. Seven WCST indices were recorded for further analysis:
- Number of categorized completed (NCC): number of runs of 10 correct responses; ranged 0 to 6.
- Trial to completed first category (TCC): total number of trials needed to achieve the first 10 consecutive correct responses; ranged 0 to 64.
- Total number of correct (TNC): trials in which the response matched the sorting principle in effect; ranged 0 to 64.
- total number of errors (TNE): total of the incorrect responses, which should be the same as the sum of perseverative and non-perseverative errors; ranged 0 to 64.
- Perseverative responses (PR): number of incorrect reactions that would have been correct for the previous category/rule; ranged 0 to 62.
- perseverative errors (PE): incorrect trials, in which the subject perseveres in reacting to an incorrect dimension; ranged 0 to 62.
- Non-Perseverative Errors (NPE): Incorrect trials that are not perseverative; ranged 0 to 64.
Aside from NCC and TCC, in the standard scoring system, high scores are representative of poor performance.
3.4. Statistical Analysis
Nonparametric statistical tests were used because the assumption of normality was rejected by the Kolmogorov-Smirnov test. The analysis of correlation between the severity of aphasia and WCST scores was tested using Spearman’s rank correlation coefficient test. Statistical analyses were performed using SPSS 18.0. Statistical significance was set at α < 0.05.
4. Results
Demographic characteristics and the lesion descriptions of the 19 enrolled subjects, and the clustering of PWA by severity (AQ scores), are provided in Table 1. The descriptive data on P-WAB-1 and the WCST indices are provided in Tables 2 and 3, respectively. As seen in Table 2, the mean score of AQ was 59.22 (SD = 13.58; ranged 39.10 to 85), that is, only mild to severe PWA participated in the current study, with very severe cases being excluded.
| No. | Age | Education in Years | Time Post Stroke, m | Loci of Lesion (Left Hemisphere) | Type of Lesion | Aphasia Severity |
|---|---|---|---|---|---|---|
| 1 | 59 | 5 | 41 | Fronto-parietal and left basal ganglia | Ischemic | Severe |
| 2 | 54 | 4 | 16 | Temporo-ocipital | Ischemic | Severe |
| 3 | 57 | 7 | 7 | Temporo-parietal | Hemorrhagic | Mild |
| 4 | 52 | 12 | 32 | Temporal and basal ganglia | Ischemic | Severe |
| 5 | 32 | 16 | 21 | Fronto-temporo-parietal | Ischemic | Severe |
| 6 | 55 | 12 | 48 | Temporo-parietal | Ischemic | Moderate |
| 7 | 42 | 12 | 38 | Fronto-temporal | Ischemic | Moderate |
| 8 | 67 | 8 | 9 | Fronto- temporal | Ischemic | Moderate |
| 9 | 69 | 8 | 6 | Basal ganglia and cerebellum | Ischemic | Moderate |
| 10 | 58 | 8 | 14 | Fronto-parietal | Ischemic | Moderate |
| 11 | 58 | 16 | 69 | Basal ganglia, left putamen | Hemorrhagic | Severe |
| 12 | 56 | 12 | 108 | Fronto-temporo-parietal | Ischemic | Moderate |
| 13 | 46 | 16 | 38 | Frontal | Ischemic | Moderate |
| 14 | 56 | 12 | 39 | Temporal | Ischemic | Moderate |
| 15 | 56 | 16 | 122 | Temporo-parietal | Ischemic | Moderate |
| 16 | 59 | 16 | 7 | Frontal | Ischemic | Severe |
| 17 | 49 | 12 | 11 | Frontal, para and periventricular, centrum semioval | Ischemic | Moderate |
| 18 | 43 | 19 | 11 | Temporo-parietal | Ischemic | Moderate |
| 19 | 63 | 12 | 6 | Frontal and parieto-occipital | Hemorrhagic | Moderate |
| Mean | 54.26 ± 8.88 | 11.74 ± 4.17 | 33.84 ± 33.62 |
Demographic Characteristics of the PWA
| P-WAB-1 score | Spontaneous Speech Content | Fluency of Spontaneous Speech | Auditory Comprehension | Sequential Commands | Repetition | Naming | |
|---|---|---|---|---|---|---|---|
| Minimum | 39.10 | 2.0 | 0 | 7.0 | 4.0 | 2.0 | 1.0 |
| Maximum | 85.00 | 9.0 | 7.0 | 10.0 | 10.0 | 10.0 | 9.0 |
| Mean | 59.22 | 5.08 | 2.64 | 8.52 | 7.68 | 5.84 | 5.31 |
| Std. deviation | 13.58 | 2.43 | 2.02 | 0.96 | 1.81 | 2.78 | 2.61 |
Descriptive Statistics of P-WAB-1 Score in PWA
| NCC | TCC | TNC | TNE.RS | TNE.PS | PR.RS | PR.PS | PE.RS | PE.PS | NPE.RS | NPE.PS | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Minimum | 0 | 0 | 24.0 | 10.0 | 5 | 1.0 | 8 | 0 | 21 | 1.0 | 5 |
| Maximum | 5.0 | 27.0 | 54.0 | 40.0 | 63 | 19.0 | 99 | 16.0 | 99 | 21.0 | 99 |
| Mean | 2.47 | 14.68 | 42.63 | 21.37 | 30.63 | 7.68 | 55.79 | 7.89 | 48.89 | 5.63 | 55.74 |
| SD | 1.12 | 7.06 | 7.03 | 7.0 | 19.05 | 4.43 | 22.67 | 3.28 | 18.09 | 4.58 | 25.05 |
Descriptive Statistics of the WCST in PWA; (RS: Raw Score; PS: Percentile Score)
The mean number of categories sorted by PWA on the WCST was 2.47 (SD = 1.12; range 0 to 5). On average, patients took 14.67 trials (SD = 7.06) to complete the first category (range 0 to 27). The mean TNC was 42.63 (SD = 7.03; range 24 to 54) (Table 3). Using age-and education-corrected norm tables in Persian (20), the patients’ overall performance as measured by TNE averaged at the 30th percentile (range 5th to 63rd percentile). Patients’ mean percentage of PR was at the 55th percentile (range 8th to 99th), mean percentage of PE was at the 48th percentile (range 21st to 99th), and mean percentage of NPE was at the 55th percentile (range 5th to 99th).
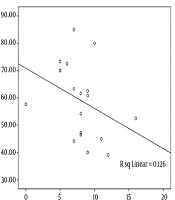
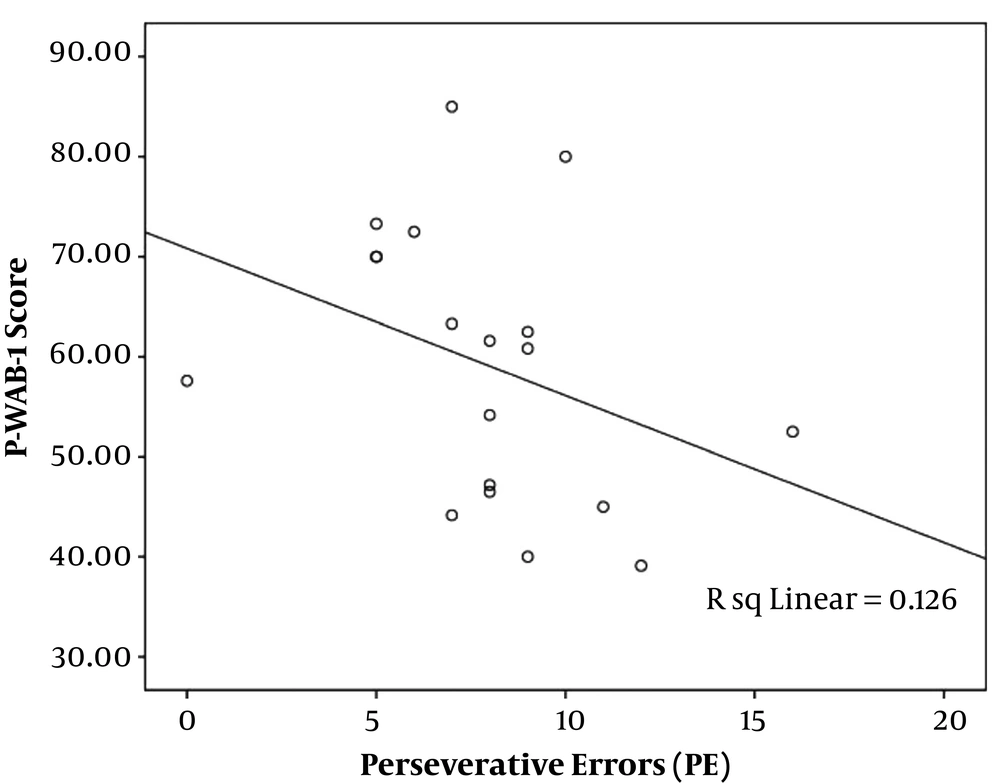
The analysis of correlation between the severity of aphasia and the WCST indices using Spearman’s rho correlations showed a significant reverse correlation between P-WAB-1 score and PE index (r = -0.48, P = 0.03) (Figure 1). In other words, patients with milder aphasia showed less PEs in WCST performance. Other WCST indices did not have a significant correlation with P-WAB-1 scores: NCC (r = -0.22, P = 0.29), TNC (r = 0.31, P = 0.19), TNE (r = -0.31, P = 0.18), and NPE (r = -0.06, P = 0.8).
Correlation coefficients were computed to measure the relationship between AQ score and the subtest of P-WAB-1 (content, fluency, comprehension, naming, and repetition) and the WCST indices (NCC, TCC, TNC, TNE, PR, PE, and NPE). Raw data was applied, instead of norm-corrected percentiles, as this study was more concerned with the associations between language and set-shifting, and less concentrated on normative data as regards to percentile cut-offs. Likewise, it was not considered desirable to edit the raw data using age- and education-corrected percentiles. The correlation coefficient obtained by Spearman showed that the fluency subtest of P-WAB-1 had a significant correlation with four indices of the WCST. As shown in Table 4, the NCC (r = 0.546, P = 0.016) and TNC (r = 0.616, P = 0.005) had a significant direct correlation, and TNE (r = -0.603, P = 0.006) and NPE (r = -0.449, P = 0.0) had a significant reverse correlation. There was no significant relationship between PE and fluency (r = -0.145, P=0.55), nor between WCST indices and other P-WAB-1 subtests.
| Spearman’s Rho | NCC | TCC | TNC | TNE | PR | PE | NPE |
|---|---|---|---|---|---|---|---|
| Fluency of spontaneous speech | |||||||
| Correlation coefficient | 0.546 | 0.624 | 0.616 | -0.603 | 0.117 | -0.145 | -0.449 |
| Sig. (2-tailed) | 0.016 | -0.120 | 0.005 | 0.006 | -0.372 | 0.55 | 0.05 |
| N | 19 |
The Correlation of Fluency of Spontaneous Speech with WCST Indices
5. Discussion
The aim of this study was to evaluate the relationship between set-shifting deficits and language difficulties in Persian-speaking PWA. For this purpose, mild to severe PWA were tested, and it was found that more severe language impairment was correlated with poor set-shifting ability. Set-shifting ability enables a person to flexibly coordinate thoughts and behaviors in order to accomplish internal goals (21). Healthy individuals, when doing the WCST, may experiment with various procedures in order to find the exact method to match the cards, yet PWA are inclined to get trapped in the card-matching job. They continue using the same directive for sorting, not caring for the feedback from erroneous responses. This continuation of an improper tactic is known as perseverative behavior. Thus, poor performance on the WCST, especially an increase in the number of PE, indicates that the PWA lacks cognitive flexibility to integrate the rule systems, and are stuck in the rules of a previous set (22). Inability to score a set may be a reflection of failure of shifting the set, and an incapability to preserve a set in the face of stimulus interference. In line with previous studies, the findings of the present study demonstrated that language difficulties and set-shifting are associated with in PWA (7, 23-25). Recent neuroimaging studies of cognitive flexibility in normal adults have identified a distributed network of fronto-parietal regions involved in set-shifting, including the inferior frontal junction, the ventro-lateral prefrontal cortex, the posterior parietal cortices, and the insula and anterior cingulate cortices (26-28). Therefore, the relationship between set-shifting deficits and language difficulties may be due to lesions in the perisylvian area in PWA.
In line with the purpose of the study, it was found that the performance on the WCST correlated with severity of aphasia, most consistently with the fluency score. It was also found that patients with lower scores in fluency completed fewer categories and had fewer corrected responses on the WCST. These subjects also had more errors, particularly in non-perseverative errors types. The WCST is known as a frontal lobe test; as well as being based on Luria’s theory of brain-functioning, following frontal lobe lesions, a disconnection between speech and motor action can lead to deficits on subtests, such as fluency, initiation, and repetition of speech (29). Besides, clinically-based researches in patients with neurogenic discourse impairments suggest a strong relationship between deficits in cognitive flexibility and competence in discourse (30). Furthermore, previous investigations have indicated a link between deficits in cognitive flexibility and difficulty in fluency, especially in verbal fluency (31). In addition, verbal fluency (semantic or phonologic) is related to the function of the frontal lobes and its adjacent areas (32-34).
In conclusion, the current study provides evidence suggesting that language difficulty, especially fluency deficit, is related to set-shifting scores in PWA. The current study may shed light on the importance of considering set-shifting deficits in the assessment and treatment of PWA. Because of the low sample size, the categorization of PWA based on the location of lesions was impossible, so that it was not possible to study if patients with frontal and patients with non-frontal lesions performed differently in set-shifting. Additional research on greater sample sizes of PWA and their classification based on frontal and non-frontal involvement, will also help uncover how the location of lesions affects set-shifting scores.