1. Background
Spinal cord injuries are among the problems that affect the lives of individuals, and it can be said that almost all individuals in the society are at risk of developing it at the same level. spinal cord injury (SCI) is one of the most serious clinical diseases where its prevalence increases year by year. Although its mortality rate has been reduced to less than 5%, the rate of disability caused by it remains high. Among these disabilities, we can mention paraplegia (1, 2). Studies have indicated that trauma to the nervous system can even cause epigenetic changes in the secretion of inflammatory cytokines (3-6). Therefore, not treating and controlling the complications of the spinal cord injuries timely will impose a lot of costs on society. Many attempts have been made to reduce the complications of spinal cord injuries, however, most of them have failed. This is probably due to the complicated responses of the nervous system to the injuries. Despite identifying many of these responses and attempting to moderate them, there is still a little success in improving these complications.
For example, the presence of two primary and secondary phases is identified in the spinal cord, and it is determined that inflammation occurs in the initial phase as well as in the first minutes after the injury, and is the known cause of many complications after the spinal cord injury (7, 8). Thus, at present, the use of anti-inflammatory treatments after SCI are among the first steps in the clinical (9-11).
Low-level laser therapy is one of the anti-inflammatory treatments that its usage to treat disease associated with inflammation has increased in recent years (12-14). Although there is not any complete protocol provided on this topic yet, the conducted researches have indicated that low-level laser is effective in the reduction of post-SCI complications (15-17).
Our previous study indicated that low-level laser (660 nm) radiation for two weeks although improves the movement and reduces tissue complications, it also leads to the entrance of fibroblasts into the lesion, and probably prevents the complete improvement of the lesion (18).
2. Objectives
Following the previous study, this study is designed to investigate whether laser radiation will affect the growth of axons over a longer period of time and will lead to the complication of the entrance of fibroblasts, or not?
3. Methods
In this study, adult male Wistar rats (N = 24) weighing 150 - 180 g were used. All animals were provided from the Empirical Studies Center of Iran University of Medical Sciences. All steps of this research project were conducted at Iran University of Medical Sciences (Iran, Tehran). The animals were kept in standard conditions of an animal house with enough access to water and food, temperatures of 21 ± 3°C, and 12 hours of darkness/light before and during the study. All ethical considerations (code: R.IUMS.REC 1395.95-03-30-26896) were carefully observed when working with animals in accordance with the rules of the National Institutes of Health for the Use of Live Animals.
Animals were randomly divided into three groups.
1. Control group: No intervention was performed on the animals in this group (n = 8).
2. Spinal cord injury group: The animals of this group did not receive any treatment after induction of spinal cord injury (n = 8).
3. Laser therapy group: The animals were exposed to low-level laser radiation for four weeks, 30 minutes after induction of spinal cord injury (n = 8).
3.1. Creating the Model of Spinal Cord Injury
Animals were anesthetized with an intraperitoneal injection of ketamine (80 mg/kg) and xylocaine (8 mg/kg). After shaving the skin of the lumbar vertebrae area and prepping with betadine, a cut was created in the middle line with a scalpel blade. After removing subcutaneous fat and muscles and exposing the lamina of vertebrae, laminectomy was performed at the L1 - T13 level of the spinal cord without damaging the Dura mater. An aneurysm clip, made by FST that provides a force of 20 g/cm2 was used to create a spinal cord injury model by the compressive method. The clip was removed after 1:30 minutes, and then the muscles and skin were stitched with suture 0.3, respectively. Post-surgical care, including the administration of ringer solution to prevent dehydration (3 mL, IP, after surgery), administration of penicillin G for four days after surgery (0.8 mg/100 g, IP), and bladder massage was performed twice a day for all animals. Three days after creating the lesion, the animals with a score higher than three in the BBB test were excluded from the study.
3.2. Functional Recovery Scoring
Each animal was allowed to move freely for four minutes in a circular-shaped area with a diameter of 90 cm and the walls of 24 cm height. Two observers independently (blind) observed the films and rated the animals according to the Basso Beattie and Bresnahan scale (BBB scale) from zero (full paralysis) to 21 (normal stepping). The mean scores of two observers were recorded as the score of each animal. One week after the injury, the BBB scored of animals were recorded. After that, animals were tested for BBB every week for five weeks, and their score was recorded.
3.3. Haematoxylin-Eosin (H&E) Staining
In order to investigate the penetration of fibroblasts around the injury site, the Hematoxylin and Eosin staining was used (19). After staining, an optical microscope (Nikon, magnification × 40) was used to examine lamels and photography. Then, the results of counting fibroblasts were quantified (18).
3.4. Bielschowsky’s Silver Staining
In order to examine the status of the axons after creating the injury and after treatment, this staining, which is based on the adhesion of the silver to the axons, was performed. Therefore, the axon was black in the images (20). Axons around the injury were counted with an optical microscope (Nikon, magnification × 20), and the results were reported quantitatively.
3.5. Low-Level Laser Therapy
In the present study, the diode CW laser with 660 nm wavelength and 100 mW power that was received as a gift from the Heltschl Company (model ME-TL10000-SK) was used. The laser was fixed on a metal rod to maintain the same radiant distance throughout the study. Thirty minutes after removing the aneurysm clip from the site of injury, which was marked before, 5 mm higher and 5 mm lower as well as the mirror of these points on the left and right of the site of injury (9 points 5 mm apart) were radiated with 660 nm low-level laser (LLL) daily. On each point, the laser was radiated for (5 seconds) in different experimental groups (overall 45 seconds). Laser radiation with this method continued for four weeks.
4. Results
4.1. Functional Recovery
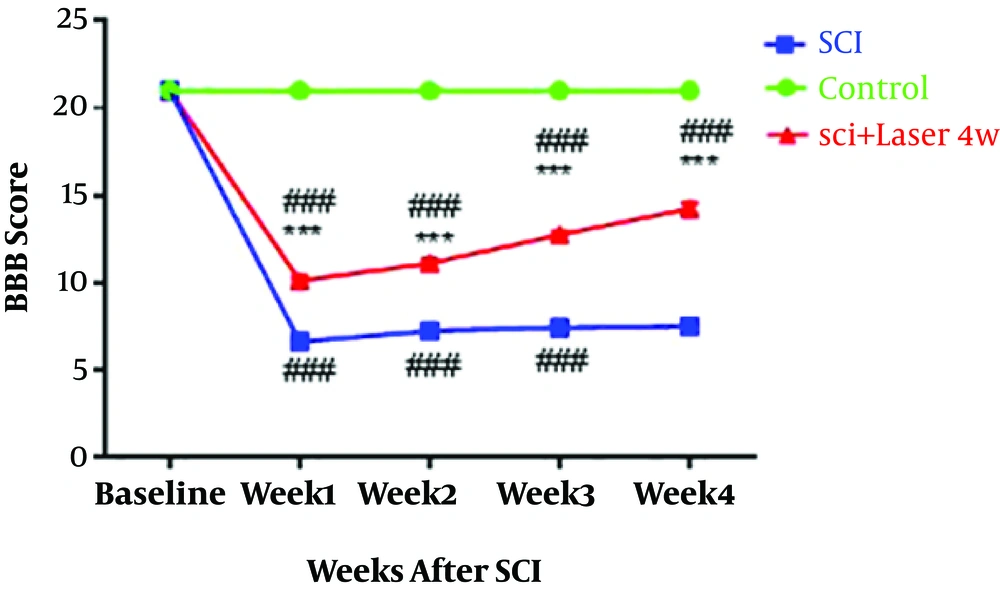
Investigating the motion status of animals in four weeks with the BBB test indicated that the induction of injury in the spinal cord causes the reduction of motion score compared to the control group (P < 0.0001, n = 8, df = 8; f = 452.5). The low-level laser radiation, which was radiated from 30 minutes after the induction of the injury to the end of the study, has produced a significant improvement in motion status compared to the SCI group (P < 0.0001); although, it still has a significant difference with the control group (P < 0.0001) (Figure 1).
4.2. Investigating the Fibroblast Penetration Around the Cavity
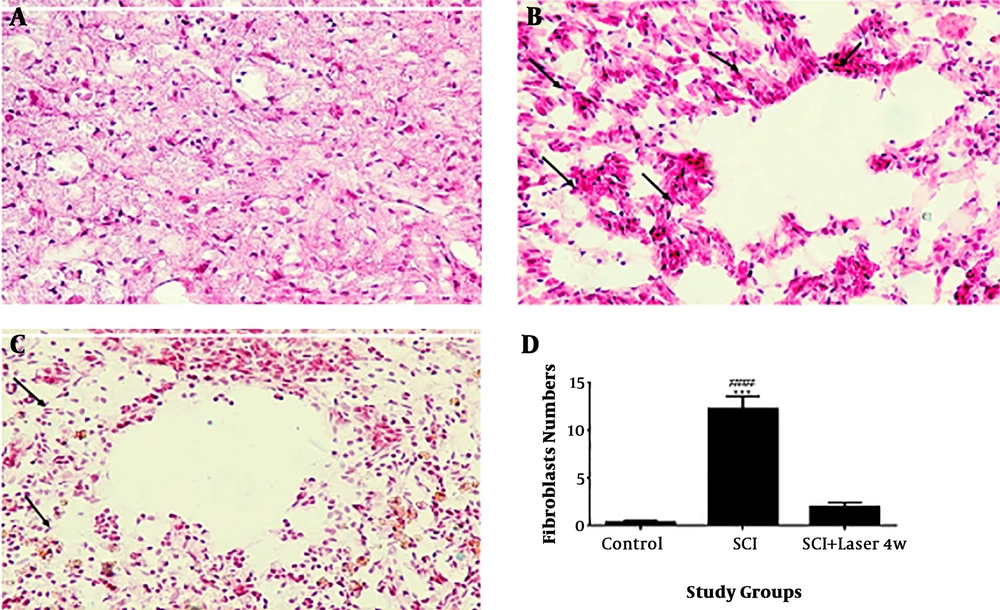
The results of Haematoxylin and Eosin staining indicated that injury induction in the spinal cord leads to penetration of fibroblasts around and inside the injury compared to the control group (P < 0.0001, n = 3, Df = 26). The accumulation of fibroblasts around the cavity is recognizable clearly (Figure 2). The presence of fibroblasts has decreased in the group treated with low-level laser radiation for four weeks. In a way that the number of fibroblasts in this group is not significantly different with the control group (P > 0.05), and there is a significant difference with the SCI group (P < 0.0001).
Quantification of fibroblasts penetration by Hematoxylin and Eosin (H&E) staining, four weeks after spinal cord injury (SCI), spinal cord transverse section (20×). Control (A); SCI (B); laser (C); quantitative assay of fibroblast penetration (D). Data are expressed as mean ± SEM. ### P < 0.001, vs control group.
4.3. Investigating the Status of Axons Around the Injury
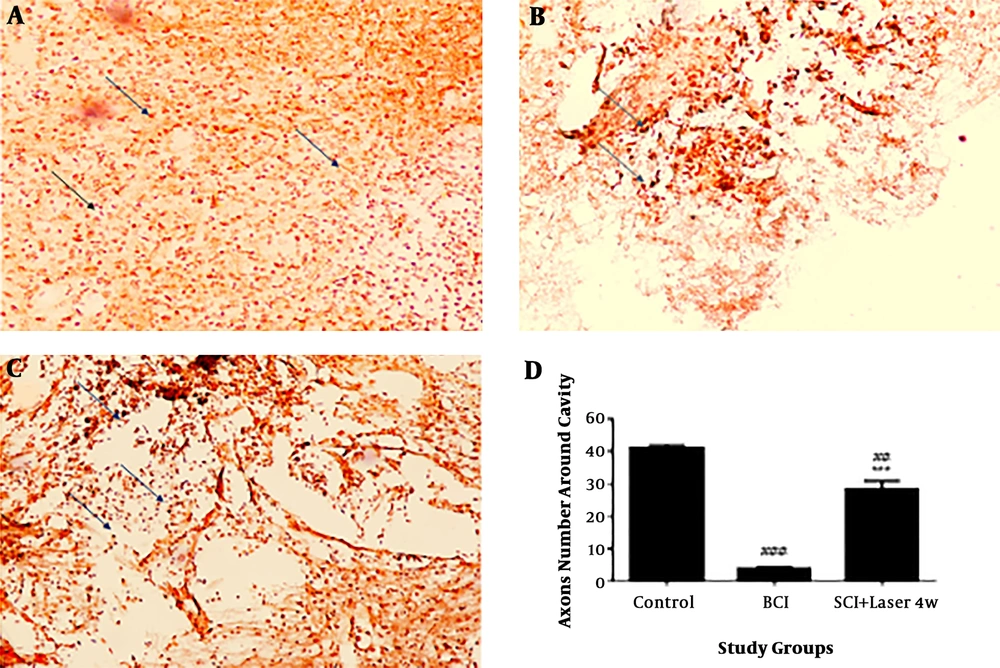
The results of a quantitative investigation of Bielschowsky staining indicated that there are few axons around the cavity due to the spinal cord injury in SCI group compared to the control group (df: 3; F = 74.19; n = 3) (P < 0.001) Figure 3. In the group treated with the laser for four weeks, the number of axons around the injury significantly increased compared to the SCI group (P < 0.0001). However, the number of axons in treated animals still has a significant difference with control group, that indicates axonal injury in these groups compared to the control group (P < 0.01).
Quantification of axons by Bielschowsky’s silver staining, four weeks after spinal cord injury (SCI) and low-level laser (LLL). The spinal cord injury has reduced the black spots (axons) and the laser treatment has prevented the loss of axons. Black points indicate axons. Control (A); SCI (B); laser for four weeks (laser 4w) (C) quantitative assay of axons (D) Data are expressed as mean ± SEM. ## P < 0.01, ###P < 0.0001, vs. control group, ***P < 0.01, vs. SCI group. Spinal cord cross-section (20×).
5. Discussion
The obtained results in this study indicated that radiation of LLL (660 nm) improved the movement of animals treated with laser, increased the number of axons around the spinal cord, and decreased the entrance of fibroblasts compared to the untreated group, although in the case of motion, difference is still observed compared to the control group.
As mentioned before, SCI complications are divided into two parts of primary and secondary phases that occur shortly after the injury to the spinal cord and lead to apoptosis and necrosis. The secretion and activation of pre-inflammatory factors such as IL1, tumor necrosis factor-alpha (TNF-α) I, and IL-6 begin to increase the minutes after trauma and following it the equilibrium between the production of ROS and antioxidants becomes unbalanced, and oxidative stress happens that lead to cell death (13). In this regard, the increase of ROS is reported around inflammation (13, 21). Apoptosis begins about an hour after the spinal cord injury in the center of the injury, and expands and continues until two weeks later on both rostral and caudal sides of the spinal cord (22). The development of inflammation activates the apoptotic cascade, which leads to the death of sensory and motor neurons and causes pain and disability in movement (21). As also observed in this study, the animals’ motion ability decreased after induction of the SCI. The obtained information from the studies confirms that the treatment should begin in a short limited time. In this study, the radiation of low-level laser (660 nm) started 30 minutes after the induction of injury to prevent the expansion of the injury as much as possible before the beginning of destructive events. Likewise, the improvement of motion observed from the first week of the treatment in this study can be the evidence to the fact that the use of LLL, a well-known therapeutic method to control inflammatory, can be a good alternative in the first line of treatment after SCI (13, 22-25). LLL reduces the secretion of cytokines and inflammatory factors, such as TNF-α, IL-1β, and IL-6 that were increased due to the inflammation (26). Likewise, our previous study indicated that LLL opposes apoptosis by reducing apoptosis through increasing the anti-apoptotic factor Bcl2, Glutathione antioxidant, and decreasing the expression of P2X3 receptors (27). There are some reports of balancing the expression of ROS in stressed cells, which can be another reason for the reduction of apoptosis (28).
In our previous study where LLL therapy was performed for two weeks, it was indicated that although a low-level laser can reduce the expression of Chondroitin sulfate, an impenetrable material forming of a glial barrier, it caused the entrance of fibroblasts into and around the injury (18). After mechanical damage to the spinal cord, the injured part is susceptible for the second phase and is separated from the healthy part by forming a glial wound (29). The fibrotic scar is the part without scar cell that consists of ECM materials. Signals activated after the injury cause the cells that are not normally present in the spinal cord to be infiltrated into the injured area (30). In this regard, the role of fibroblasts and inflammatory cells can be addressed. The fibroblasts enter from the environment and convert into resident cells and introduce inhibitory extracellular matrix (ECM) combinations into the spinal cord. The presence of fibroblasts decreases glial mobility and makes glial wounds more penetrable for the entrance of axons, create physical barrier and reduces the hope for improvement (30, 31). Therefore, the increase in the entrance of fibroblasts, which was observed in the previous study, was a negative point of using LLL. We presumed that this could justify why the cavity did not reduce, which we observed in the LLL group. It seems that an increase in the duration of laser radiation from two weeks to four weeks prevented the entrance of Lymphocytes. As observed in another study, the increase of treatment duration from one week to two weeks significantly reduced the number of lymphocytes (18, 32).
In a short time after the spinal cord injury, the axonal connections are eliminated, the axonal demyelination is created, and axons and arteries are damaged, which totally results in loss of signal transduction and reduction of neurological function. The results of this study also indicated that after SCI, few axons are observed around the injury, while laser radiation prevented the reduction of axons. The previous findings indicated that a number of supra-spinal neurons survive from the damage. These neurons that are in the non-regenerative stage are appropriate candidates for stimulation by treatments that affect nerve tissue regeneration (22). There is the probability that the axons observed around the SCI area after four weeks, are of the axon types that are regenerated by the stimulation of LLL. On the other hand, several studies have indicated that LLL causes regeneration in environmental damage (33-35).
The reduction in the number of fibroblasts and the increase in the number of axons, along with motion improvement, can cause the hope to reduce the complications of spinal cord injury. However, further studies are needed to investigate the effects of LLL on complications such as pain.
5.1. Conclusions
The results of this study indicated that low-level laser (660 nm) radiation that was initiated 30 minutes after SCI, 45 seconds every day for 4 weeks, was able to improve the BBB score and increased the number of axons around the injury, and on the contrary to the previous study, prevented the entrance of fibroblasts. Therefore, there is the probability that the anti-inflammatory effects of LLL are time-dependent, and the treatment should continue until the destruction process exists.