1. Background
The neuronal activity in the brain is accompanied by different, complex physiological activities. Neurons need nutrition to produce energy and generate activation patterns in the brain. Glucose, like oxygen and other materials, is transmitted through blood vessels and capillaries of the brain to the active metabolic neurons to increase the cerebral blood flow (CBF) in the activated area of the brain. The amount of oxygen metabolism, CMRO2, is significantly increased during the neuro-functional activity (1). This increase in oxygen consumption during neuronal activity reduces tissue oxygenation, which is compensated by increasing the amount of O2 by increasing CBF and brain blood volume (2-5). Therefore, a brain region is activated when the CBF increases, which, in turn, reduces deoxygenated hemoglobin (deoxy-Hb) and increases oxygenated hemoglobin (oxy-Hb) (6). Hence, changes in CBF can demonstrate high-resolution brain function.
Near-Infrared Spectroscopy (fNIRS) is a portable method for non-invasive and non-ionizing evaluation of brain activity, which acts through measuring hemodynamic changes in a cortical region based on optic principles. The fNIRS is a tool designed to detect changes in the concentration of oxy-Hb and deoxy-Hb in the blood (7, 8). This effective and indirect method of brain neuroimaging uses optical waves to monitor hemodynamic responses related to brain activity given that nervous activity and vascular responses are closely coupled and are called “neurovascular coupling”. In this method, two or more different wavelengths are used to examine brain activity. Different wavelengths (in the range of 700 - 900 nm) are separately absorbed by oxy-Hb and deoxy-Hb. Based on the calculation of changes in light intensity, the location of increased concentrations of oxy-Hb and deoxy-Hb can be determined in the brain tissue and an activation map can be obtained. This method provides many details about the physiological and functional properties of the brain by measuring the changes in oxy-Hb and deoxy-Hb.
The fNIRS is used for functional examination and imaging of hemodynamic changes in the brain in a healthy condition and a variety of pathologies. The fNIRS is a research method with known limitations (low spatial resolution, low depth of penetration, noise sensitivity, etc.) that is not widely used in clinics. It is suitable for individuals and patients who are not capable of performing other imaging modalities. Today, infrared spectroscopy is widely used to assess visual, auditory, motor, and cognitive stimuli (9-12). It has been proven that each area of the brain is responsible for certain movement control, but the function of many areas of the brain is unrecognized. Neurological studies performed in inhuman mammals have shown that unique neurons in the PMC (primary cortex), M1 (primary motor cortex), and the cerebellum are responsible for encoding the direction of movement (13-17). When the movement is in the preferred direction, the frequency of neurons activity is maximum and when the motion is in the opposite direction, it is reduced. Based on a simple model, the activity of the unique neurons that make preferential coding corresponds to a cosine function. Moreover, based on this model, when moving to the preferential direction, cosine function has the maximum neuronal function. When we take a 90-degree angle, the cosine function is half and when we take 180 degrees, the cosine function places in the base position. It is believed that a large number of these sensitive neurons encode the actual directions of our movements to move in a vector-mediated manner (14, 18). Hence, a number of studies have investigated the relationship between muscular activity and brain activity detected by fNIRS (19).
Some studies showed that it is possible to differentiate the brain activity pattern in the movement of hands and feet (20). In a study, Sato et al. found that the movement of the arm can be distinguished between two directions with an accuracy of 87.5% (19). On the other hand, Yeo et al. in 2013 conducted a study to investigate the differences in brain cortical activity between proximal and distal joints of upper extremities. The results of the HBO and HBT group analysis indicated the activation of the left SM1, PMC, and PFC during right shoulder movements. In contrast, the activation of the left SM1 was only observed during right-hand movements (21).
2. Objectives
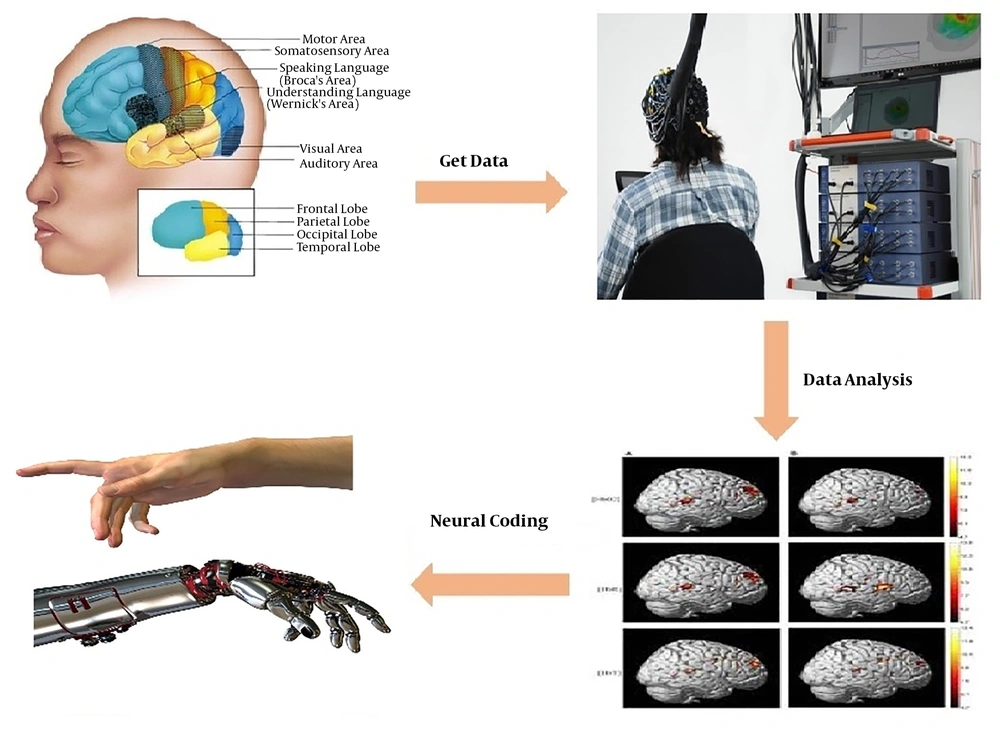
For this reason, we conducted a study aiming at analyzing the cerebral cortex during simple hand movement using fNIRS data. Figure 1 shows the extraction steps of brain activation maps with fNIRS data.
3. Methods
3.1. Participants
We recruited 10 right-handed volunteers in this experiment. The participants had no history or current symptoms of neurological and psychosocial illnesses and no history of using drugs that would stimulate the nervous system. The participants were fully aware of all stages of the study and they signed written consent forms. Moreover, all the tests carried out in this study were approved by the Ethics Committee of Tehran University of Medical Sciences (TUMS), Tehran, Iran. We explained the whole procedure of the task to the volunteers. The protocol of the human study was approved by the Local Ethics Committee of TUMS (approval number: IR.TUMS.MEDICINE.REC.1396.3968).
3.2. Functional Near-Infrared Spectroscopy Recording
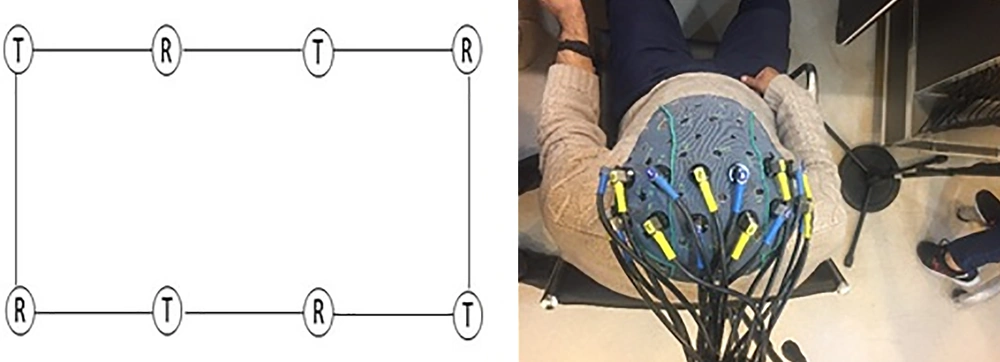
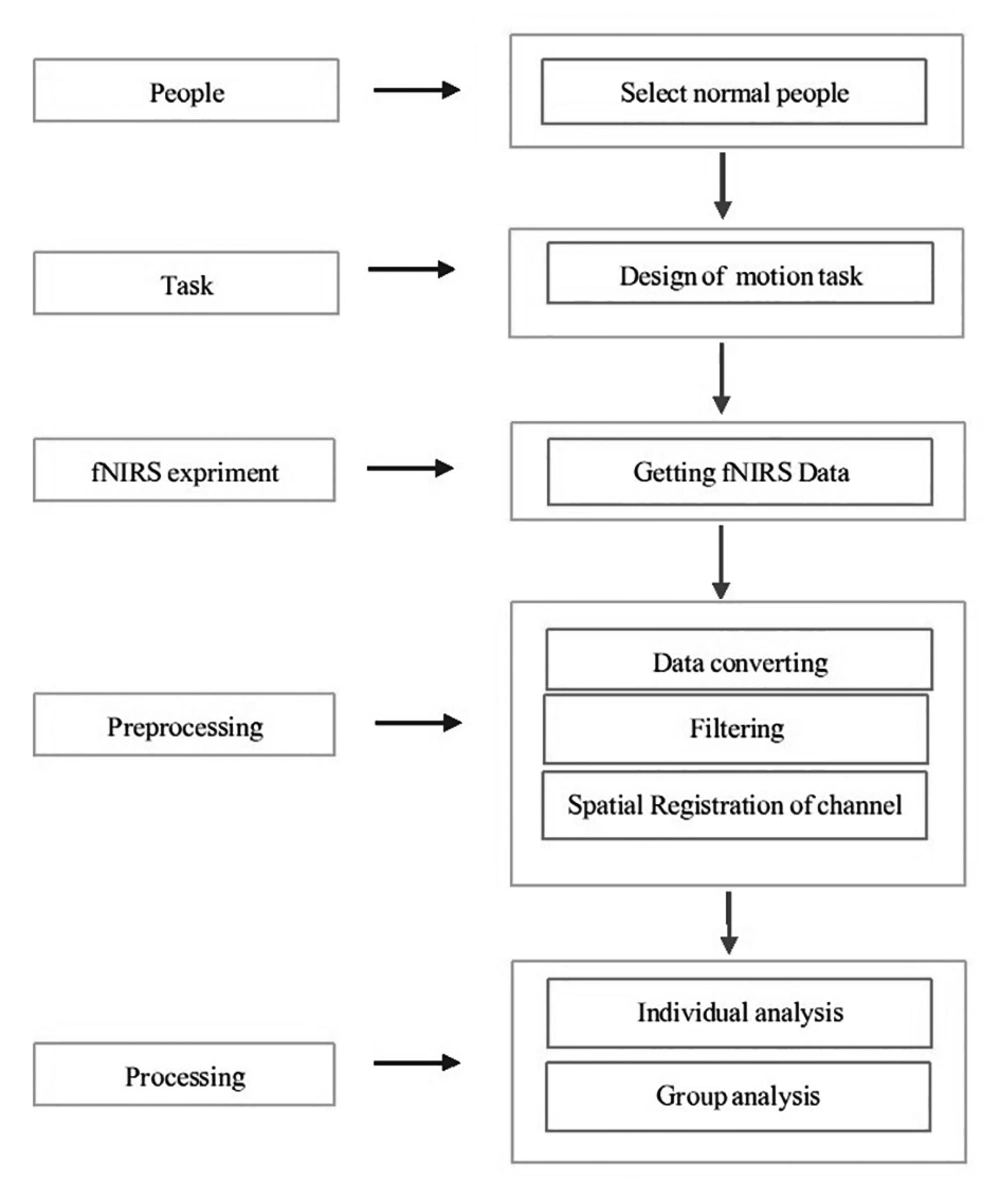
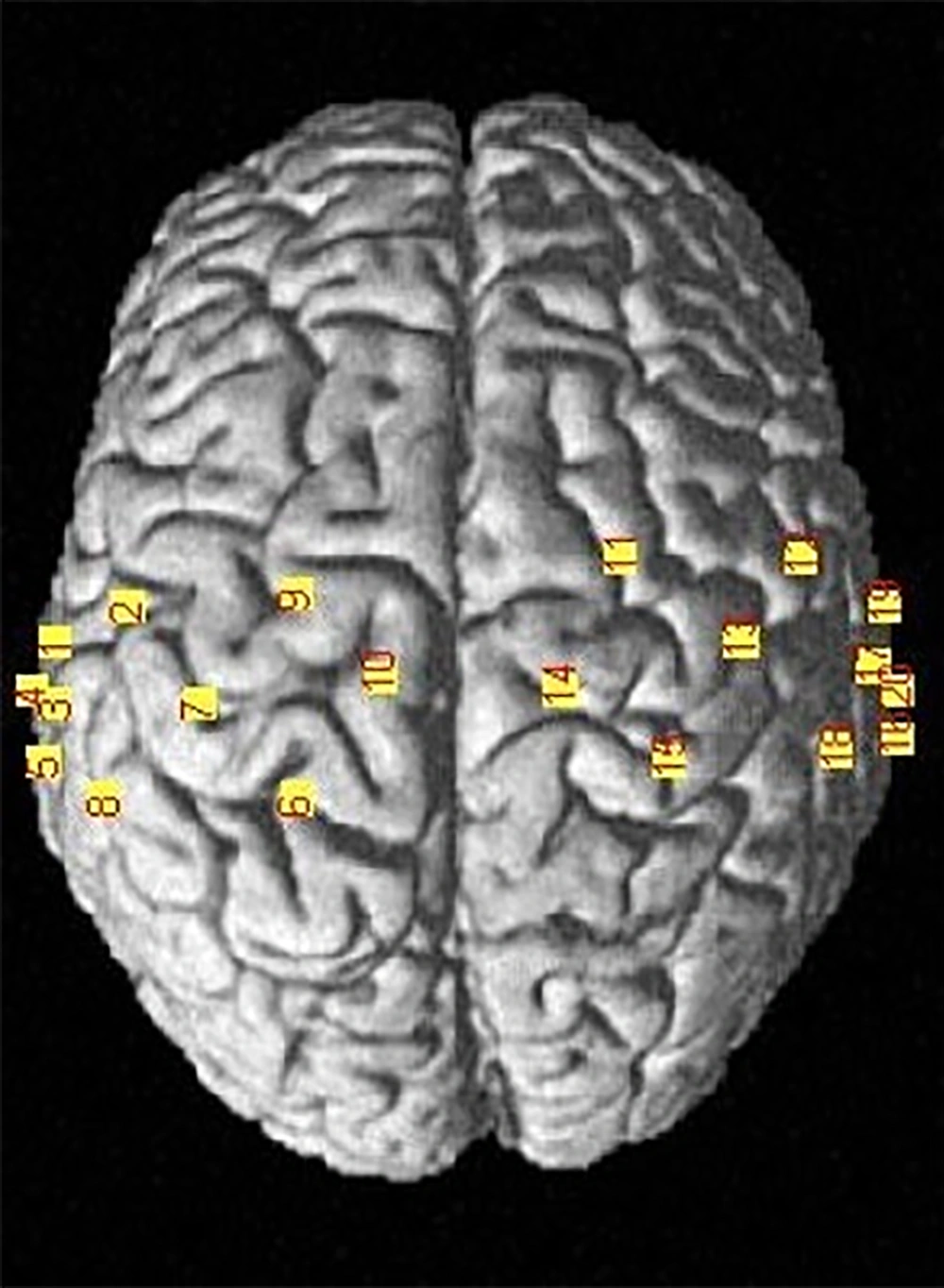
The data were collected using a 48-channel fNIRS MR-compatible device (OxyMon fNIRS from Artinis) with two wavelengths of 855 nm and 765 nm. For this experiment, eight transmitters and eight infrared light receivers were used to generate a total of 20 channels (Figure 2). The distance between the transmitter and the receiver was 30 mm and the sampling frequency was set at 10 Hz. Figure 3 shows the flow work in this study.
3.3. Experimental Design
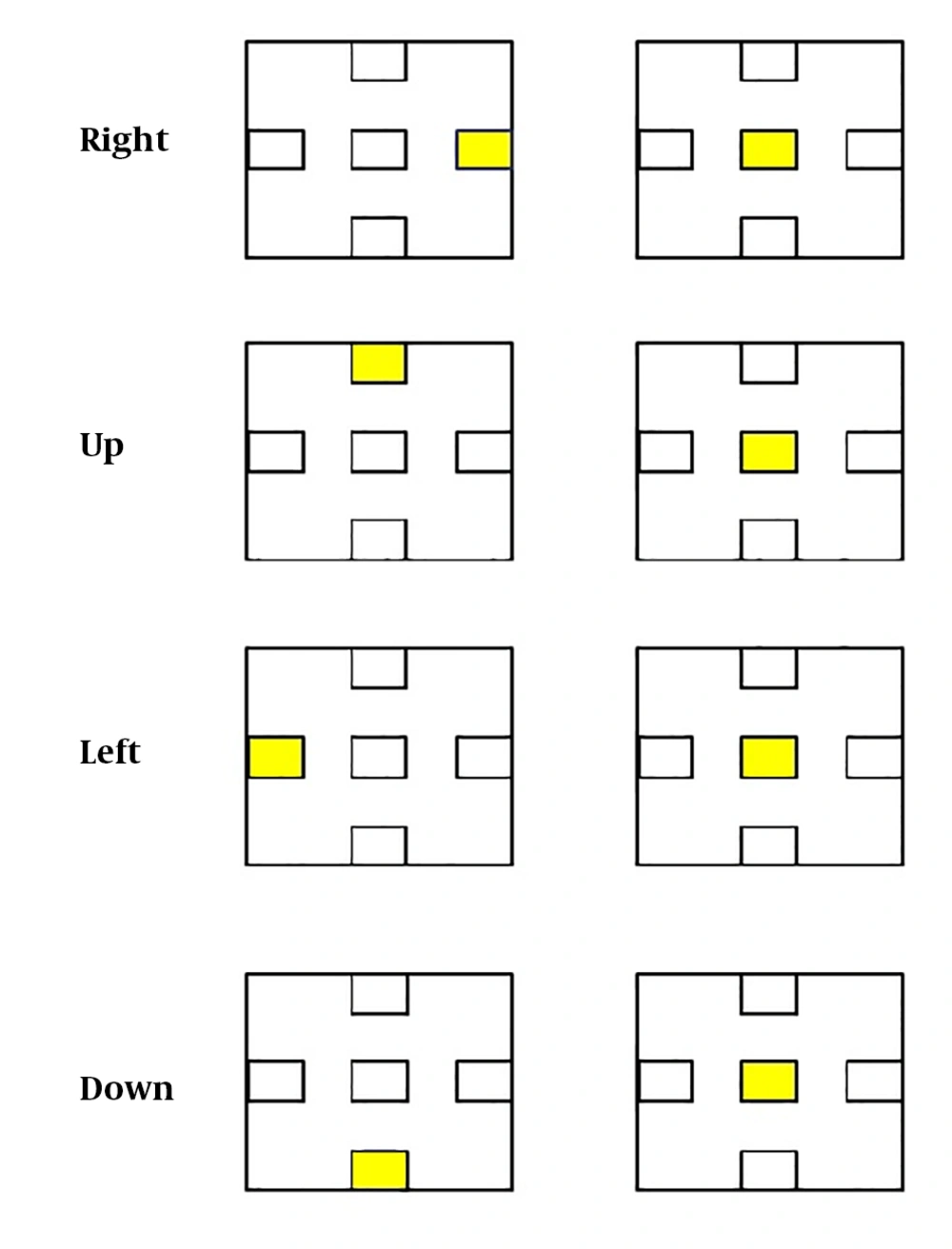
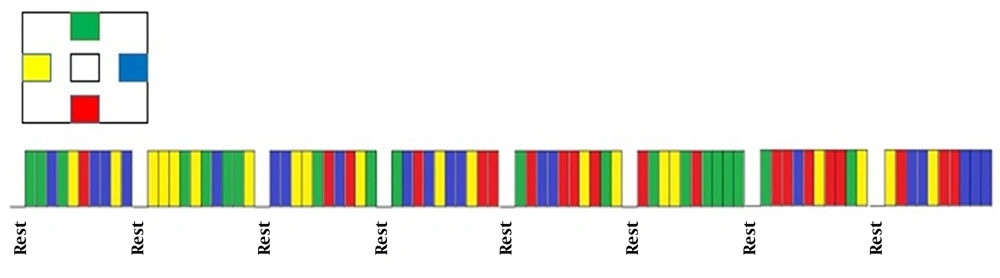
During the tests, the participants kept their body steady while performing a step-tracking task by moving their right wrist in four directions in the sequence of right-left and up-down. These movements were accompanied by extension, flexion, radial deviation, and ulnar deviation. For this purpose, some images (Figure 4) were shown to individuals showing the directions of movements; the person had to move his hand in the randomly selected direction showing in the image after the subject pointed in each specific direction (up, down, left, and right), the center image was displayed, where the subject was supposed to place his hand at the center, and the hand was placed in the basic state. The person performed the movements as quickly as possible. The duration of staying in each angle was 3 s and the duration of holding the hand in the center was 2-4 s. Thus, every step-tracking movement lasted 6, 5, to 7 s. The test started with a 4-s rest, and at the end of every 10 step-tracking movement, there were 4 s of rest. The movement task in the present research lasted 496 seconds, where the subject pointed 20 times in each direction (up, down, left, and right) during the task period. (Figure 5).
3.4. Data Analysis
The data were analyzed using NIRS_Statistical Parametric Mapping (NIRS_SPM) that was run on MATLAB R2016a software. Initially, the data format recorded by the OxyMon fNIRS device was changed to the readable format for NIRS_SPM. The changes in the concentration of oxy-Hb and deoxy-Hb were estimated based on changes in the optical density recorded by the device. In the next step, the fNIRS channels were placed on the head. At this stage, the locations of the fNIRS channels were displayed on the Montreal Neurological Institution (MNI) atlas (Figure 6). On the other hand, because the brain could be found in different shapes and geometric properties, at this step, each individual’s brain anatomy was mapped onto the MNI atlas, and transformed to the standard space. After this step, the steps of level-1 analysis, estimation, and group analysis were taken, respectively. After completing these steps, we used the data such as the number of test modes, the number of scans, and the time of applying different stimuli to design matrixes and contrasts required for calculations. Finally, to view the desired results, the symmetric T-contrast matrixes were designed and went to be used. In the next step, the P value was selected at the level of 0.05 and the brain activation map was obtained during hand movement. NIRS_SPM uses the functions and algorithms of the SPM toolbox. In this software, a random-effects analysis method was used for group analysis. Finally, the type of contrast (T-contrast) was used for observation and the FWE collective error control method was used to obtain the final results.
3.5. Filtering
The data were analyzed using NIRS_SPM in MATLAB software. The t-statistic SPM maps were calculated at a significance level of 5%. To analyze fNIRS data, HbO levels were used to calculate cortical activity because HbO is a sensitive marker indicating hemodynamic changes during neuronal activity. The Gaussian smoothing filter was used to remove the noise generated by the fNIRS machine. During the data analysis process, the wavelet-MDL-based detrending algorithm was used to correct the fNIRS signaling of respiration, heart rate, and motion.
3.6. General Linear Model and Functional Near-Infrared Spectroscopy Data
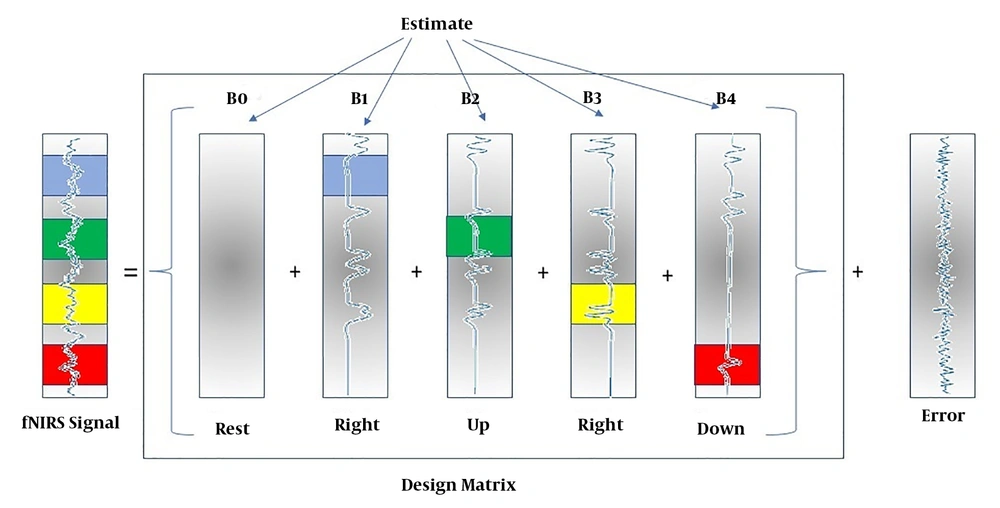
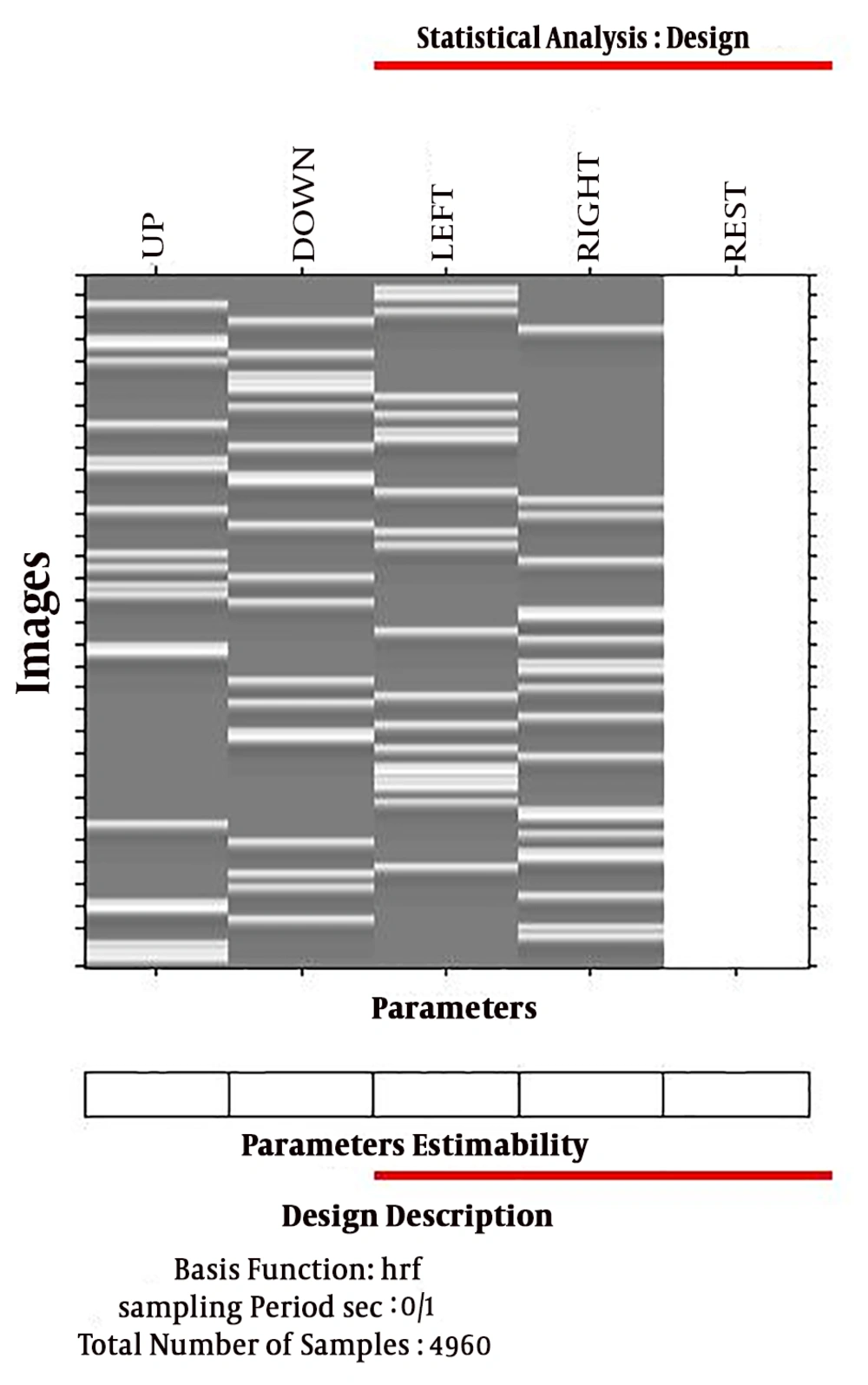
The General Linear Model (GLM) method was used to calculate the amount of increased or decreased activity relative to the rest mode. For this reason, some rests were designed in tasks. The GLM algorithm used for this study is shown in Figure 7. The GLM method is one of the models based on the fNIRS data analysis model, in which a data model is initially developed and after data analysis, it is concluded that how much the assumed model fits the data. In this method, a specific model is designated for the excitation function. It can be judged if the data and model are matched; then, it can be stated whether the data are stimulated by that function or not. This linear model is expressed as follows:
Y = X. β + ε
Observed = Predictors * Parameters + Error
where y (t) is the time series of each voxel, x (t) is the function of the excitation model of each voxel, β is the estimated parameter for x (t), and e is the model error.
In this notation, matrix X represents the design matrix containing the predictor time courses as column vectors. The beta values appear in a separate vector b. The term Xb indicates matrix-vector multiplication. The GLM Model for TASK testing is used as follows:
In fNIRS design matrices, all conditions are modeled as predictors in the design matrix including the baseline (rest, right, left, up, and down) condition. We would not include the rest condition (recommended); the design matrix would not be multi-collinear and the four beta weights B1, B2, B3, and B4 would be interpretable as increases or decreases in activity relative to the baseline signal level modeled by the constant term (Figure 8).
4. Results
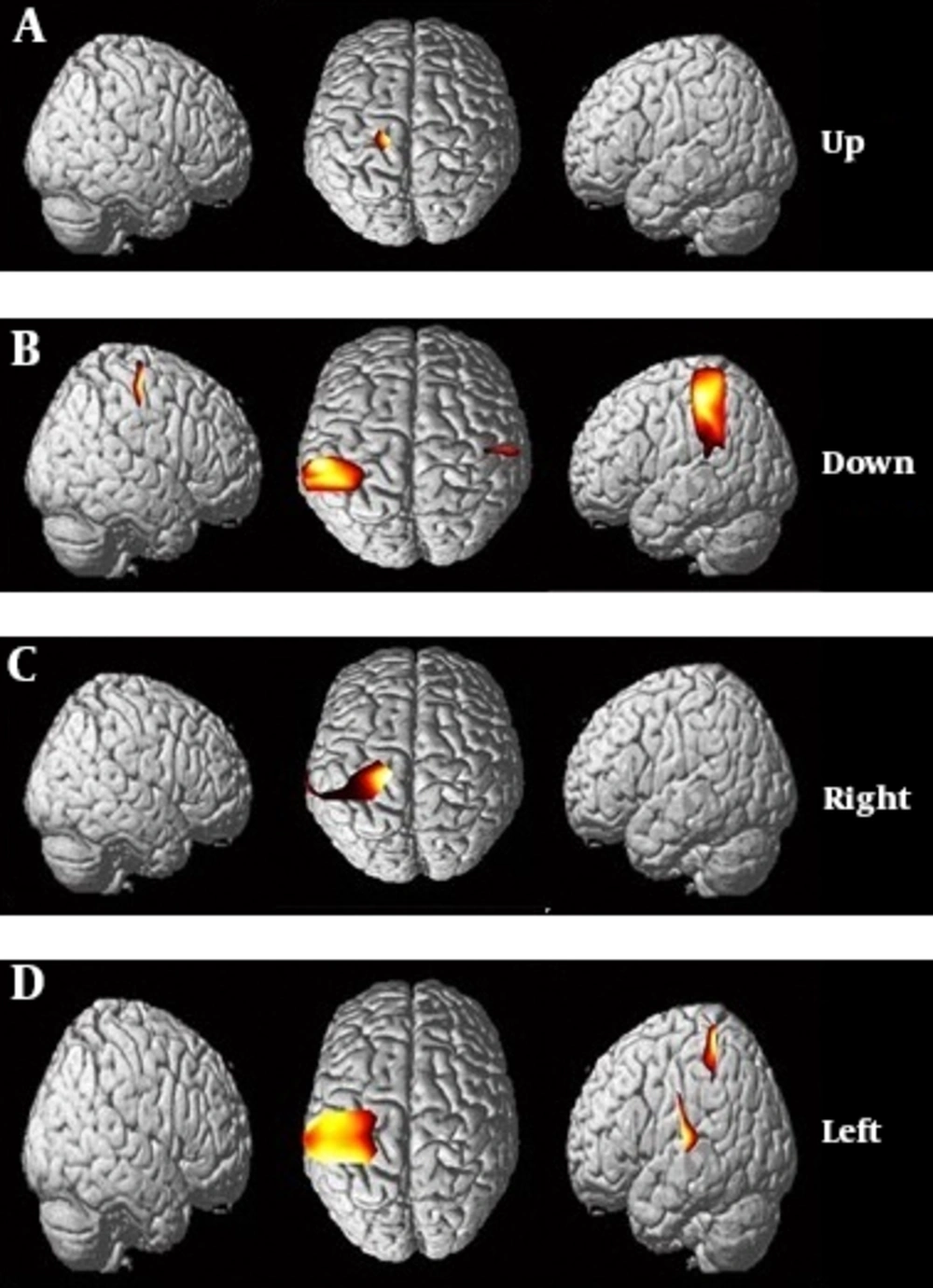
In this study, separate activation patterns were obtained for hand movement in each direction. The brain activation maps were observed visually in certain areas in the motor cortex. The most activation observed in the primary motor cortex M1 (Broadman area 4). The pattern of brain activity was different in the four states of wrist movement, which distinguished between hand movement patterns in different directions. In the motion of the right wrist, regardless of directions, brain activity was observed contralaterally in the motor cortex. However, there was a significant difference in the focus of the maximum activation of the brain between the four main directions (0, 90, 180, and 270) (Figure 9).
Results from NIRS_SPM. The activation pattern obtained from the motor cortex of the brain, by means of hand motion. A, activation map measured in upward movement; B, activation map measured in downward movement; C, activation map measured during hand movement to the right and D, activation map measured during hand movement to the left.
The results showed that the activity of the brain was higher in the motion of the wrist to the left than in the motion in other directions. The activation associated with motion in the direction of zero degrees (upward) was the highest in the middle of the motion. Activation associated with motion in a 270º direction (right) extended from the midpoint of M1 to the lateral motor cortex.
The brain activity map recorded by the channels of fNIRS showed that the cerebral activity patterns of the brain in hand movement to up, down, left, and right were schematically (Figure 9) and spatially (Table 1) different.
| Wrist Movement Direction | Brain Region | Broadman Area | MNI Position | Channel Number | T Value | ||
|---|---|---|---|---|---|---|---|
| z | y | x | |||||
| Up | |||||||
| Primary motor cortex | 4 | 79 | -25 | -13 | 10 | 2.98 | |
| Down | |||||||
| Primary somatosensory cortex | 2 | 51 | -45 | -60 | 8 | ||
| Primary motor cortex | 4 | 68 | -27 | -43 | 7 | 2.63 | |
| Primary somatosensory cortex | 2 | 35 | -28 | -68 | 3 | ||
| Primary motor cortex | 4 | 68 | -27 | -43 | 2 | ||
| Right | |||||||
| Primary motor cortex | 4 | 79 | -25 | -13 | 10 | ||
| Pre-motor and supplementary motor cortex | 6 | 73 | -10 | -28 | 9 | ||
| Primary somatosensory cortex | 2 | 51 | -45 | -60 | 8 | 2.88 | |
| Primary motor cortex | 4 | 68 | -27 | -43 | 7 | ||
| Superior temporal gyrus | 5 | 10 | -39 | -70 | 5 | ||
| Primary somatosensory cortex | 2 | 35 | -28 | -68 | 3 | ||
| Left | |||||||
| Pre-motor and supplementary motor cortex | 6 | 73 | -10 | -28 | 9 | ||
| Primary somatosensory cortex | 2 | 51 | -45 | -60 | 8 | 2.39 | |
| Primary motor cortex | 4 | 68 | -27 | -43 | 7 | ||
| Primary somatosensory cortex | 1 | 74 | -44 | -28 | 6 | ||
| Primary somatosensory cortex | 2 | 35 | -28 | -68 | 3 | ||
In upward movement, in Broadman area 4, the activity signal significantly increased and activity map was obtained. Additionally to Broadman area 4, signal activity was increased in Broadman area 2 in the downward movement. In wrist movement to the right direction, Broadman areas 4, 6, and 2 were activated. In wrist movement to the left, Broadman areas 4, 6, and 1 were activated.
5. Discussion
The present study aimed to investigate the capability of a neural imaging system including near-infrared spectroscopy (fNIRS) in evaluating the activation of the cerebral cortex during a motor activity that humans perform daily. In fact, fNIRS examines hemodynamic changes in the brain as a measure of neural activity. To achieve this goal, we monitored the changes in the activity of the motor cortex during performing motor tasks by the fNIRS multichannel machine.
The current study showed that activation related to the motion direction was separable spatially in the cerebral motor cortex and the highest activity was recorded in the primary M1 motor cortex. In moving upward, the primary motor cortex and premotor cortex were activated while in moving downward, primary somatosensory cortex was also activated in addition to the primary motor cortex (Table 1). In the move to the left, the premotor cortex was also activated in addition to the primary motor cortex and primary somatosensory cortex; however, in moving to the right, the activity was recorded only in the primary motor cortex, which indicated the presence of separate neurons focusing in the cerebral cortex to control hand movements. One of the strengths of our study was the placement of channels on the motor cortex to prevent the registration of unrelated and extracorporeal brain activities occurring outside the scope of the motor cortex related to the hand during the course of the activity. Therefore, the recorded activity was only related to the hand movement and it was free of additional information.
In fact, we could extract the neuronal activity pattern of the hand moving in different directions. The results of the study are consistent with the results of previous studies in inhuman mammals showing that neurons were regulated sensitive to the direction of motion in the brain's primary motor cortex (22). Similarly, these results are consistent with recent findings of neural coding in the human brain (16). On the other hand, the results of our study showed that we could successfully design a specific method to evaluate brain changes and distinguish between these changes in the movement of the wrist in different directions. Optical imaging, especially fNIRS, has attracted researchers’ attention as a way of evaluating cortical activity in the last decade. Our results confirmed that the differentiation between various directions of hand movement is possible by fNIRS, which is consistent with the results of the study by Sato et al. (19). The presence of directional activation in the cerebral cortex contributes to the development of the notion that this part of the cerebral cortex, in addition to controlling the movement of different parts of the body, contributes to the processing of complex information. Besides, designing a test pattern for recording the activity of cerebral motor cortex in this study was to test the block design, which was useful for the purpose of the study and provided a higher SNR value.
The results showed that the activation pattern obtained from moving downward is very similar to the activity pattern in moving upward. The greatest activity was achieved in moving to the left, which is due to the higher nerve function in moving to the left relative to moving in other directions (Figure 9). Performing this movement task showed that neurons in the cerebral motor cortex could control the movement of the right wrist.
This study provided evidence that there is a significant relationship between the performance of motion tasks and activation changes in the cerebral motor cortex (Figure 9). The results of many previous studies showed that the pattern of cortical activity is associated with the motion of joints, but according to our knowledge, this study is the first study to examine and distinguish between the patterns of brain activity in the motion of the wrist. In addition, the results showed that fNIRS is a convenient and practical tool for research in recording hemodynamic changes in the brain following increased neurons activation as a result of performing a wrist motion task.
Cowper-Smith et al. (23) conducted a study to demonstrate the adaptation of neuronal cells of the human brain cortex during right-hand movement in 12 healthy right-winged subjects. In this study, the participants pointed to a target shown using a joystick group, which was approximately 9 cm long and 1.3 cm wide with 8º rotation maximum (flexion/radial deviation/ulnar deviation extension). Eventually, this study showed that when the participants performed repetitive motions in one direction, the BOLD signal increased in the M1, SMA, and PMC regions.
The study showed that the control of movement direction is done in the M1, SMA, PMC, and the cerebellum of the human brain. Our study also aimed to determine the activity of the cerebral cortex during the motion of the wrist using fNIRS imaging. The results showed that the motion of the wrist in different directions could activate the primary motor cortex, primary somatosensory cortex, premotor cortex, and supplementary motor cortex. The results of this study also showed that fNIRS imaging produces similar results to fMRI findings while fNIRS has high resistance to motion artifacts, high temporal resolution, safety, low price, and high motion and displacement capacity during imaging.
The most important limitation in this study was the mere selection of participants who were healthy and young with no symptoms of neuropsychiatric disorders and excluding older people and patients who may have significant differences with healthy young people in the pattern of activity. On the other hand, due to the low penetration depth of the light, it was hard to record brain activity in deep layers of the cerebral cortex. We believe that our results can be useful for future studies of motion control. In addition, our findings can be a theoretical basis for rehabilitation interventions in people with brain damage.