1. Background
Migraine is a common, disabling, chronic lifelong disorder with moderate to severe headache attacks (1). Migraine mostly affects women, as its prevalence in women (5% - 25%) is reported to be about three times that of men (2% - 10%) (2, 3). It should be noted that migraine often remains undiagnosed (4), and only 3% - 5% of migraineurs undergo prophylactic treatment (5). What gives priority to migraine treatment is that this disorder, unlike other chronic diseases, is predominantly concentrated in healthy young people, especially women (6). The relationship between migraine and menstruation is reported in about half of women with this disease (7). Pure menstrual migraine has a specific definition in the International Classification of Headache Disorders (ICHD3 beta), and is considered to be a headache attack that occurs on days -2 to +3 of menstruation in at least two out of three consecutive menstrual cycles.
On the other hand, menstrual-related migraine is the impression of migraineurs who have the attacks on days -2 to +3 in addition to other attacks during a menstrual cycle (8). Generally, headache attacks in this period are more severe, last longer, and are more resistant to medication (7, 9, 10). Several drugs have been proposed for short-term prophylaxis of menstrual-related migraine attacks, including Triptan (11), non-steroidal anti-inflammatory drugs (12), hormones (13), and so on, each with both benefits and complications. To improve the quality of life of migraine patients, preventive therapies for recurrent attacks are required. In fact, these treatments are prescribed to reduce the frequency, severity, and duration of headache attacks (14). Preventive treatments of migraine are commonly used for patients who suffer from frequent or debilitating headaches or do not respond to other treatments and also for patients in whom common abortive therapies are contraindicated (14, 15). Prophylactic drugs are usually much less effective on menstrual-related migraine attacks than on other attacks during a month (16). B-group vitamins are often effective in preventing headache attacks (17). These vitamins, as an important part of the diet, appear to affect the clinical signs of migraine (18). For instance, the role of pyridoxine, folate, and cobalamin has been reported in the severity and frequency of migraine attacks (19). The association between the combination of B-group vitamins and migraine has been revealed in some limited studies (20-22). Neurobion is a combination of neurotropic vitamins B1, B6, and B12. This compound containing 100 milligrams of thiamine (vitamin B1), 100 milligrams of pyridoxine (vitamin B6), and 1000 micrograms of cyanocobalamin (vitamin B12) is prescribed as an intramuscular injection. As the effects of the combination of vitamins B1, B6, and B12 on menstrual-related migraine attacks were not previously addressed systematically, we designed a single-arm open-label study to investigate the efficacy and safety of Neurobion for prophylaxis of menstrual-related migraine attacks. In this study, we tested the hypothesis that the combination of vitamins B1, B6, and B12 might reduce the severity of menstrual-related migraine attacks, which are usually the most severe type of migraine attacks in women.
2. Methods
2.1. Study Design
This quasi-experimental (before and after) study was conducted on women with menstrual-related migraine, who were referred to a headache clinic in Tehran, Iran, from March 2017 to June 2018. The study was approved by the Institute Ethics Committee of the Tehran University of Medical Sciences. All the patients gave their written consent. This was an investigator-initiated single-center study with no external financial support.
2.2. Patient Selection
The patients were recruited from the out-patient service of Sina Hospital. The diagnosis of migraine was based on the International Headache Society (ICHD3 beta) criteria (8). The inclusion criteria were female gender, active and regular menstruation, age between 18 and 52 years, migraine attacks occurring between days -2 and +3 of menstruation in at least two of last three menstrual cycles, suffering from migraine for more than a year, and at least one migraine attack other than the menstrual-related one per month. Patients with liver or kidney failure and malignancy, patients changing their prophylactic migraine drugs during the last two months before enrollment, and pregnant patients were excluded. Moreover, patients already consuming group B vitamins or multivitamin supplements were excluded.
2.3. Clinical Evaluation
The patients underwent a thorough clinical evaluation by an experienced neurologist inquiring for migraine characteristics (frequency and severity), comorbidities, and drug history. The migraineurs were grouped into two diagnostic categories: (1) episodic migraine (< 15 attacks per month) and (2) chronic migraine (≥ 15 attacks per month with at least eight days of headaches with migraine characteristics or response to triptans, for at least three months) (23). During the study, the patients were asked to record the intensity, date, frequency, and duration of their headaches on a printed dairy form designed by the senior researcher (24). The rating of headache intensity was carried out based on a 10-point visual analog scale (VAS), where 10 indicates the most severe pain. The improvement percentage of headache severity based on the 10-point VAS was defined as the primary outcome of the study.
2.4. Intervention
There was a base time period of one month for each patient to record characteristics of her migraine attacks, including the time and severity score of her menstrual-related attacks. Participants meeting the inclusion criteria entered the study. They were instructed to inject each ampoule, containing 100 mg of vitamins B1 and B6 as well as 1000 μg of vitamin B12, one week before starting menstruation, and repeat the injection for three consecutive months. At the end of the three-month intervention period, the completed diary forms were collected from the patients.
2.5. Statistical Analysis
The data were processed in SPSS version 19 (SPSS Inc., Chicago, IL). Statistical significance was defined at P < 0.05. Descriptive statistics were presented as mean ± SD for continuous data and frequency (percentage) for categorical variables. The Student's t test was used to determine any significant difference between the two subsets of migraine, while the Paired t-test was applied for intra-group comparisons between baseline and end of intervention. Moreover, the chi-square test was used to compare categorical data.
3. Results
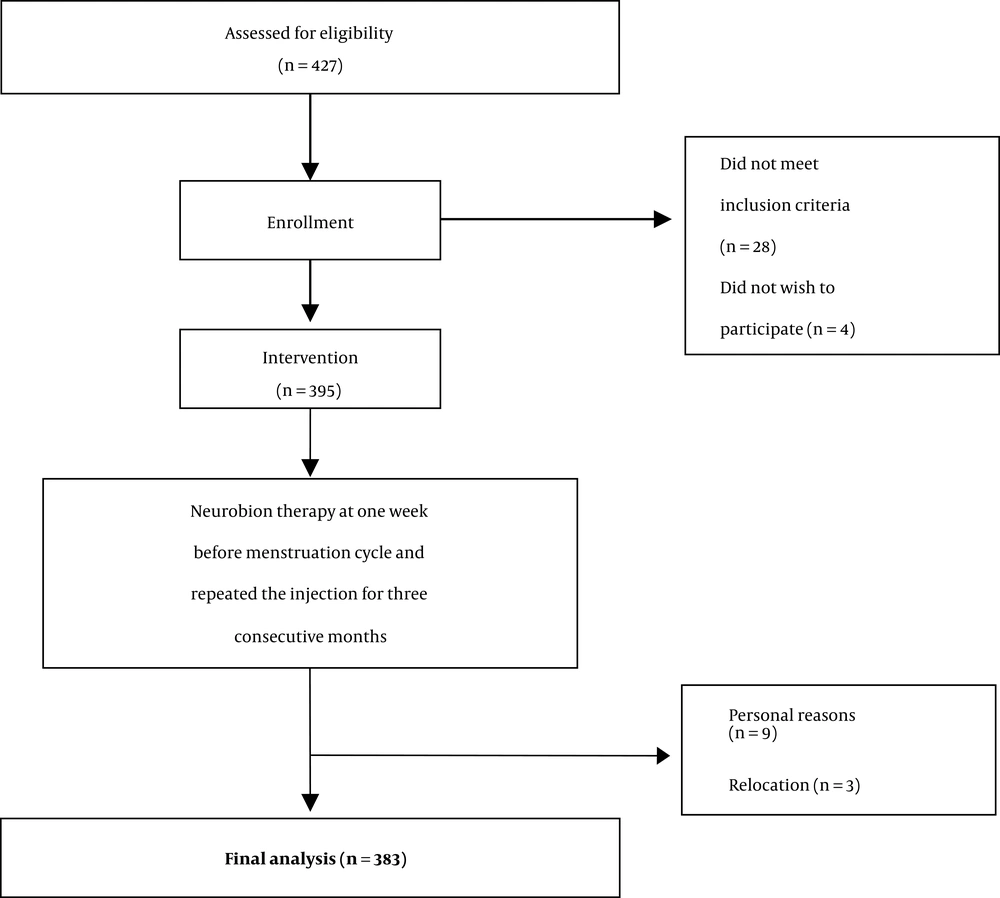
A total of 395 eligible patients were identified who met the inclusion criteria and received Neurobion for migraine prophylaxis. Of the total 383 patients completing the study, 169 were diagnosed with chronic migraine headache and 214 with episodic migraine. The mean age of the patients was 34.7 years (ranging between 18 - 50). Moreover, the mean age of patients with chronic and episodic migraine was 35.3 and 34.2 years, respectively (P = 0.203). Table 1 shows the baseline characteristics of patients completing the intervention.
aValues are expressed as mean ± SD.
bP values based on student t-test.
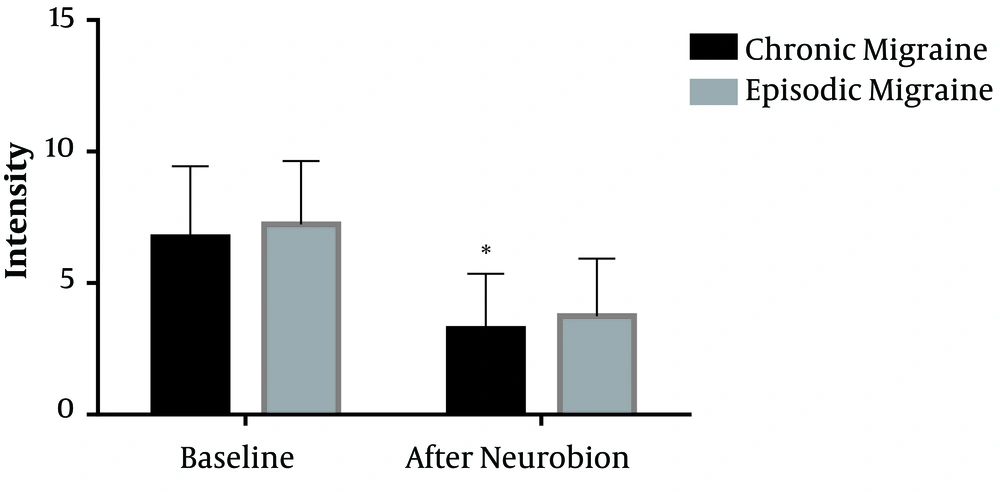
Among patients with chronic migraine, the mean intensity of menstrual-related migraine attacks was reduced from 6.7 on the 10-point VAS to 3.2 (P < 0.001). In addition, the mean severity of menstrual-related migraine attacks was reduced from 7.2 to 3.7 in patients with episodic migraine (P < 0.001) (Figure 1). However, there was no significant difference in the reduction of headache severity between the two groups of migraineurs (P = 0.985). Sixty-one percent of patients with chronic migraine and 53% of patients with episodic migraine experienced more than 50% improvement in the severity of menstrual-related migraine attacks (Table 2) based on the 10-point VAS evaluation. There was no significant difference in the reduced severity of headaches in response to Neurobion between patients with chronic and episodic migraine (P = 0.257).
| Quartile 1 | Quartile 2 | Quartile 3 | Quartile 4 | |
|---|---|---|---|---|
| Chronic migraine | 70 (41.4) | 33 (19.5) | 38 (22.5) | 28 (16.6) |
| Episodic migraine | 81 (37.9) | 32 (15) | 62 (29) | 39 (18.2) |
At the end of the trial, 383 out of the 395 patients completed the intervention period, indicating an overall 97% compliance, and no side effects were reported. A flow chart of the study design is reported in Figure 2.
4. Discussion
Various drugs have been proposed for prophylaxis of menstrual-related migraine attacks (25-28). However, the side effects of such drugs sometimes prevent any further consumption (29). In the present study, the impact of the combination of vitamins B1, B6, and B12 was evaluated on the intensity of menstrual-related migraine attacks, with notable positive effects. Great efforts are being made to understand the pathophysiology of menstrual-related migraine attacks. Clinical studies indicate a strong association between estrogen levels and migraine attacks in women. Abrupt drops in estrogen appears to trigger headache attacks, only before menses (30, 31). Another proposed mechanism is the increase in NO production, which appears to be due to the increased activity of L-arginine pathway in the luteal phase (32). It has also been observed that serotonin levels in this phase are reduced in women with menstrual-related migraine attacks (33). Several factors, including genetic and environmental factors, contribute to the onset of migraine attacks, although the main cause of migraine is still unknown. Migraine can be attributed to several factors, including a mutation in the MTHFR gene, increased serum levels of homocysteine and nitric oxide (NO), mitochondrial malfunction, and metabolic enzyme reduction (34). Moreover, the association was shown between some nutrient deficiencies and genetic factors such as flavoenzyme 5, 10-methylenetetrahydrofolate reductase (MTHFR), especially the C677T variant with high plasma levels of homocysteine (35). It has been revealed that homocysteine, by inducing endothelial nitric oxide production, a factor of vasculopathic risk, can be involved in the pathogenesis of migraine (36). Our study was conducted to investigate the efficacy and tolerability of a combination of vitamins B1, B6, and B12 for prophylaxis of menstruation-related migraine. Women diagnosed with menstrual-related migraine, both chronic and episodic types, began Neurobion therapy one week before the menstruation cycle and repeated the injection for three consecutive months. Among patients with chronic and episodic migraine, the severity of menstrual-related attacks was reduced. The present study was the first attempt to evaluate the effects of a combination of vitamins B1, B6, and B12 on the severity of menstrual-related migraine attacks. As mentioned, it was proposed that NO could be one of the causative factors in migraine attacks. It appears that B12 can be a NO-scavenger. In the study of P-HM van der Kuy et al., It has been shown that intranasal hydroxocobalamin (OHB12), as a NO-scavenger, can be effective in migraine prophylaxis. One mg intranasal hydroxocobalamin daily improved migraine attack frequency (37). The exact mechanism of action of thiamine in the pathogenesis of migraine has not yet been clearly specified, but its role in mitochondrial function is guaranteed. Thiamine is an important coenzyme in energy production in mitochondria. A disruption in the mitochondrial function, which leads to impairment in oxygen metabolism, may be associated with migraine pathogenesis (20). Prakash et al. conducted a study to investigate the effect of intravenous thiamine on chronic migraine. They reported two female patients with chronic migraine. Both the patients showed a low blood thiamine level. In this study, improvement of headache was observed in both groups of patients following intravenous thiamine administration. This intervention also reduced the frequency and severity of headache attacks (38). Regarding pyridoxine, previous studies have stated that the administration of pyridoxine to migraine patients may lead to improved vascular functions and subsequent reduction of migraine attacks (39, 40). However, the precise mechanism that can explain the improvement of migraine symptoms following pyridoxine has not yet been determined. One of the proposed mechanisms is the reduction of the serum homocysteine concentration following pyridoxine administration. Sadeghi et al. conducted a double-blind randomized clinical trial to investigate the effects of supplementary pyridoxine on the severity, frequency, and duration of migraine attacks as well as headache diary results (HDR) in 66 patients with migraine with aura. In this study, the intervention group received 80 mg of pyridoxine per day, and patients in the control group received placebo for 12 weeks. The results of this study indicated that supplementation with pyridoxine significantly reduced the severity and duration of headaches, but failed to significantly alter the frequency of migraine attacks (41). In addition, since migraines mainly affect women, it is believed that fluctuation in estrogen levels which controlled by the estrogen receptor 1 polymorphisms has an important role. The decremented level of estrogen in the last days of a menstrual cycle appears to be the main cause of menstrual-related migraine attacks (42). Increasing the cellular concentration of the active form of pyridoxine can significantly reduce the response of the gene transcription when estrogen binds to its receptor. In fact, pyridoxine can reduce the biological response of the body to estrogen through modulating estrogen-induced gene expression (35). Generally, group B vitamins contain various substances that contribute to the prevention of migraine attacks. Therefore, regular supplementation of these vitamins can have additional benefits in reducing the incidence of migraine attacks (20).
4.1. Study Strengths and Limitations
The main strength of our study was the evaluation of a safe vitamin supplement for menstrual-related migraine headaches that are usually severe, prolonged, and sometimes, disabling. However, the patients were not followed up after the completion of the trial. Thus, it is not clear how long the effect of the current intervention would last after the treatment has been stopped. Another significant limitation of this study is that no sham group was studied.
4.2. Conclusions
Based on the results of the present study, a combination of vitamins B1, B6, and B12 is a safe and effective drug for prophylaxis of menstrual-related migraine attacks and reduction of headache severity. Randomized controlled trials should be conducted to assess the effects of the vitamin B family members on menstrual-related migraine attacks. Furthermore, animal studies are suggested to explore the effects of these vitamins on migraine headaches.