1. Background
Cell-cell communication is essential for a variety of both normal physiological and pathologic processes (1). Besides secreted soluble factors, cells can communicate via extracellular vesicles (EVs), collectively known as secretome (2). EVs contain any cell-derived membranous vesicles released into microenvironment that transport bioactive molecules between cells (3). EVs, especially exosomes, are considered as a new vehicle to transfer genetic material from donor to recipient cells. Exosomes have nano-spherical, membrane-type structure (30 - 150 nm in diameter) containing some proteins such as Hsp70, Hsp90, GTPases, annexins, flotillin, CD9, CD63, CD81, and CD82 (4).
Mesenchymal stem cells (MSCs) are adult stem cells with a multipotent differentiation capacity. In recent decades, clinical trials in the regenerative medicine are focused on MSCs due to their special features such as homing to damage tissue, producing paracrine factors with anti-inflammatory properties, and modulating immune responses (5). Although a variety of clinical trials are so far conducted in the field of regenerative medicine suggesting the feasible and safe clinical use of MSCs (6-8), cell-based therapies still remain issues, including poor cell survival and gross genetic alterations (9). Mentioned concerns and the findings that MSCs can produce large amounts of non-immunogenic and safe EVs compared with other cells as well as MSCs secretome could attribute similar effects to MSCs leading to diversion of the attention of cell-based therapies into cell-free therapies. Possibility of EVs sterilization, their off-the-shelf employment, and proper storage condition might be the advantages of EV-based therapies compared to cell-based therapies (10-12).
Recently, MSC-EVs, especially MSC exosomes, are important intermediates to exert the biological functions of MSCs (13). In addition, it is shown that MSC-derived exosomes perform a wide variety of MSCs functions (14). Accordingly, MSC-derived exosomes evince a prominent potential in stem cell-free therapeutic strategies.
The contents of exosomes vary depending on the tissue source and environmental context of the donor cells (15); hence, a suitable cell source should be used for a specific clinical purpose. The current study focused on the use of human endometrial MSCs (hEnMSCs) to isolate hEnMSC exosomes.
The lining (endometrium) of the uterus is a highly self-renewing tissue with the specific characteristics of fast and highly regulated angiogenesis. Endometrium is a new source to access the adult stem cells with immunoprivileged characteristics that can be obtained without anesthesia (16). Due to their immunoprivilege, isolating hEnMSCs is a new perspective for cell-based therapies as well as a new candidate for regenerative medicine (17).
In recent decades, therapeutic angiogenesis received increasing attention especially in regenerative medicine, playing crucial roles in many physiological processes including wound healing and tissue repair (18). MSCs and MSC-derived exosomes are increasingly studied, experimentally and clinically, for therapeutic angiogenesis and several studies consider the clinical benefit for cutaneous wound healing, ischemic/stroke treatment, and tissue repair (19-22). Interestingly, Alcayaga-Miranda et al., reported that hEnMSCs exhibit superior angiogenetic properties compared to bone marrow stem cells (BMSCs) (23). In addition, the beneficial effects of hEnMSCs on heart failure and critical limb ischemia are confirmed by several preclinical studies (24-26). However, to date, the biological role of hEnMSC-derived exosomes is still unknown.
2. Objectives
The current study aimed at investigating the effect of hEnMSC-derived exosomes on proliferation and angiogenesis of human umbilical vein endothelial cells (HUVECs). For this purpose, the hEnMSCs and then hEnMSC-derived exosomes were isolated and characterized; cell proliferation (MTT) assay, wound healing assay, and tube formation assay were applied to examine the effect of hEnMSC-derived exosomes on the ability of HUVEC proliferation, migration, and angiogenesis, respectively.
3. Methods
3.1. Isolation and Characterization of hEnMSCs
Isolation and characterization of hEnMSCs were carried out according to the method previously described by Heidari-Keshel et al., in the laboratory (27). Briefly, endometrial tissue specimens were obtained either after hysterectomy or by biopsy from healthy women with recurrent abortions in Imam Khomeini Hospital in Tehran, Iran. The protocols of the current study were approved by the Ethics Committee of Tehran University of Medical Science. The specimens were placed in pre-warmed (37°C) Hank’s medium (HBSS; Invitrogen, Carlsbad, CA) containing 1% (v/v) penicillin-/streptomycin (Sigma, USA) and 1 μg/mL amphotericin B and delivered to the cell culture laboratory and washed three times with pre-warmed Hank’s medium containing 1% penicillin-/streptomycin. Tissue was dissociated using collagenase type I (1 mg/mL; Sigma, USA) at 37°C in 5% CO2 for 45 - 60 minutes. The resulting suspension was passed through a 70-µm cell strainer once, and then through a 40-µm cell strainer twice. Cells were transferred to T25 culture flasks containing 3 mL pre-warmed (37°C) culture media (Gibco Dulbecco’s modified eagle medium (DMEM)-F12, 15% fetal bovine serum (FBS), 1% penicillin-/streptomycin). The media were changed every three days.
The phenotype characterization of the adherent cells after third passage (hEnMSCs) was performed by flow cytometry (FACCS Calibure, BD bioscience San Jose, CA, USA) for surface markers with the fluorescein isothiocyanate- or phycoerythrin - or peridinin chlorophyll protein complex-conjugated anti-(CD90, CD146, CD105, CD31, CD34, and CD45) monoclonal antibodies.
3.2. Isolation of hEnMSC-Derived Exosomes and Determination of Its Concentration
At 80% confluency, hEnSCs were washed once with PBS and incubated with serum-free medium for 60 minutes at 37°Cin 5% CO2. Then, the medium was replaced with exosome-free medium (EFM) containing serum; 24 hours after incubation, the conditioned medium containing exosomes derived from cultured hEnMSCs was collected and used for purification by differential centrifugation as follows: first, three successive centrifugations (Beckman Coulter X-14R centrifuge) at increasing speeds were performed to remove cells and debris (300 ×g for 10 minutes, 2000 ×g for 10 minutes, 10000 ×g for 30 minutes, respectively, and the pellet was thrown away at each stage, all centrifugations were performed at 4°C). To pellet the vesicles corresponding to exosomal size, the final supernatant was next ultracentrifuged at 100000 ×g for 70 minutes at 4°C (Beckman Coulter XL90 ultracentrifuge). To eliminate protein contamination, the resulting pellet was resuspended in a large volume of 0.2 nm filtered PBS and repelleted at 100000 ×g for 70 minutes at 4°C. The freshly isolated exosomes were resuspended in phosphate-buffered saline (PBS) and aliquoted (10 µL). Some of the exosomes were used to determine the concentration and characterization; the remaining aliquots were stored at -20°C.
The protein concentration of freshly prepared exosomes was measured by Bradford assay and standard curve was produced using bovine serum albumin (BSA) in triplicates at 0, 10, 20, 30, 40, and 50 µg.
3.3. Characterization of hEnMSCs Exosomes
The different characterization technologies used to assess size and morphology of the isolated exosomes included dynamic light scattering (DLS), electron microscopy, and Western blotting.
3.3.1. Dynamic Light Scattering
Size distribution of exosomes was evaluated by DLS using a Zetasizer Nano ZS (Malvern Instruments, Malvern, UK) based on the manufacturer’s guidelines.
3.3.2. Electron Microscopy
To characterize and visualize exosome morphology and particle sizes, scanning electron microscopy (SEM) and transmission electron microscopy (TEM) were used. A 10-µL aliquot from the freshly exosome suspension was fixed for SEM by 2.5% paraformaldehyde. After dilution with distilled water, 5 µL of the solution was poured in a glass slide drop wisely. After dehydration with 75% ethanol and drying, the sample was covered with a thin layer of gold and visualized by SEM (QUANTA SEM system; FEI Company, Hillsboro, OR, USA).
To visualize exosomes using TEM, another 10 µL aliquot from the freshly exosome suspension was fixed by 1% glutaraldehyde; 5 µL of it was dripped onto a carbon-coated grid. After drying at room temperature and washing twice with PBS, the sample was stained with 1% uranyl acetate and then examined under Leo 906E (Zeiss) TEM.
3.3.3. Western Blotting
Western blotting analysis was performed to assess the exosomal marker protein CD63. After extraction of total exosomal proteins using RIPA buffer (radioimmunopercipitation assay) and separating on 12% sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE), proteins were transferred to nitrocellulose membrane. Membrane was blocked overnight at room temperature with blocking solution (5% milk and 0.05% Tween-20 in PBS), and then was incubated with primary anti-CD63 monoclonal antibody (Santa Cruz Biotechnology, Dallas, Texas, USA) for 2.5 hours. Subsequently, membrane was washed with PBS in triplicates and incubated with secondary horseradish peroxidase (HRP)-conjugated antibody (SinaClon, Tehran, Iran) for two hours at room temperature and then washed with PBS. The corresponding bands were visualized using chemiluminescent detection system.
3.4. Fluorescent Labeling and Tracking uptake of Exosomes by HUVECs
HUVECs were purchased from Pasteur Institute of Iran (Tehran, Iran) and cultured in DMEM containing 10% FBS. To investigate whether the hEnMSC-derived exosomes could be transferred into HUVECs, exosomes were labeled with 1,1’-dioctadecyl-3,3,3’,3’-tetramethylindocarbocyanine perchlorate (DiI dye) and then incubated with HUVECs. To label exosomes, 6 µL of 10 µM DiI dye stock solution was added to 1 mL of freshly isolated exosome sample and incubated for 60 minutes in dark at room temperature. Excess unincorporated dye was removed by twice ultracentrifugation (120000 ×g for 90 minutes at 4°C) and washing exosomes pellet with PBS. Final exosome pellet was resuspended in 1 mL PBS, aliquoted and stored at -80°C. A small amount of exosomes was used to analyze the efficiency of exosome labeling using fluorescence microscope.
DiI-labeled exosomes were incubated with HUVECs (%70 cell confluence in 24-well plate) for 24 hours. Subsequently, internalization of DiI-labeled exosomes by HUVECs was detected with fluorescence microscope.
3.5. Evaluation of the Effect of hEnMSCs Exosomes on HUVECs
The effect of hEnMSC-derived exosomes on the proliferation, migration, and angiogenesis of HUVECs was investigated using MTT cell proliferation assay, scratch wound healing assay, and tube formation assay, respectively.
3.5.1. MTT Cell Proliferation Assay
A total of 5 × 103 cell/well was seeded in a 96-well plate and cultured with complete media (DMEM plus 10% FBS). After 24 hours, cells were treated in triplicates with 0, 25, 50, 100, 150, and 200 µg/mL of hEnMSC-derived exosomes. To investigate endothelial cell proliferation, MTT assay was carried out after 24 and 48 hours of treatment. Medium was removed, cells were washed with PBS, and pulsed with 10 µL/well 3-(4,5-dimethylthiazolyl-2)-2,5-diphenyltetrazolium bromide (5 mg/mL MTT in PBS, Sigma, USA). Resulting purple MTT formazan crystals were dissolved in 200 µL DMSO and incubated at room temperature for 30 minutes. Absorbance was read at 570 nm.
3.5.2. Scratch Wound Healing Assay
HUVECs were cultured overnight in six-well plates at a 2 × 105 cell/well density. Wound was created in each well by scratching using a sterile 100 μL pipette tip. Subsequently, the cells were washed with PBS and treated in triplicate with 0, 25, 50, 100, 150 and 200µg/mL hEnMSC-derived exosomes. Images of phase-contrast microscope were captured at 0, 6, 12, 18, and 24 hours after treatment. ImageJ (NIH, Bethesda, MD, USA) was used to measure the area of the scratch in each well.
3.5.3. Tube Formation Assay in Matrigel
To examine the effect of hEnMSC-derived exosomes on endothelial cell angiogenesis, 2 × 104 HUVECs were seeded onto Matrigel-coated 96-well plates (Corning, Matrigel®,catalog number: 354234) and treated with 0, 25, 50, 100, 150 and 200 µg/mL hEnMSC-derived exosomes and cultured in M199 with 10% FBS for 24 hours. The culture images were captured by an inverted microscope with digital camera and then the images were analyzed using ImageJ version 1.44p (NIH, USA) for colony scoring and measuring the length of tubes.
3.5.4. Quantitative Real-Time PCR
A total of 2 × 105 cell/well was seeded in six-well plates, cultured overnight, and then treated in triplicates with 0, 25, 50, 100, 150 and 200 µg/mL hEnMSC-derived exosomes. Total RNA was extracted 24 hours after treatment using RiboEX-LS total RNA solution (GeneAll Biotechnology, Seoul, Korea) as recommended by the manufacturer. The cDNA was synthetized using PrimeScriptTM RT Reagent Kit (Takara, Tokyo, Japan). RealQ Plus 2x Master Mix Green (Ampliqon, Herlev, Denmark) was used to measure the gene expression levels in untreated and exosome-treated cells by Corbett Rotor-Gene 6000 HRM Real Time PCR Machine. The expression of the housekeeping phosphoglucomutase 1 (PGM1) gene was used as normalizer. The relative expression levels of proliferation-related (Ki-67 and PCNA) and angiogenesis-related genes (Tie2, Ang2, Ang1, and VEGF) were measured using qRT-PCR.
3.6. Statistical Analysis
Statistical analysis of data was performed using SPSS version 18.0 (SPSS Inc., Chicago, IL, USA). Relative quantification analysis of target genes between the groups was performed with the relative expression software tool (REST 2009). Scratch and tube formation data were analyzed using NIH ImageJ software version 1.44p (NIH, USA). Graphs were plotted using Prism Software (version 6.00 for windows, GraphPad Software, La Jolla, California, USA). Data were expressed as mean ± standard deviation (SD). Statistically significant differences were presented after analysis of variance (ANOVA). A P value of < 0.05 was considered statistically significant. Each point or column represents the mean ± SEM; P < 0.05 (*), P < 0.01 (**), P < 0.005 (***).
4. Results
4.1. Characterization of Isolated hEnMSCs and hEnMSC-Derived Exosomes
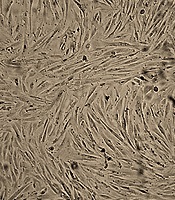
The hEnMSCs were isolated from human endometrial tissue as described in the methods section. The spindle-shaped, fibroblast-like hEnMSCs (Figure 1A) were characterized by flow cytometric for cell surface markers. The cells were positive for mesenchymal cell markers such as CD90, CD105, and CD146 and negative for hematopoietic cell markers such as CD31, CD34, and CD45 (Figure 1B).
Validation of isolated hEnMSCs. A, hEnMSCs culture at passage 3. The adherent hEnMSCs were exhibited spindle-shaped, fibroblast-like appearance. B, flow cytometric analysis showed positive expression of CD90, CD105, and CD146 (mesenchymal cell markers) and negative expression of CD31, CD34 and CD45 (hematopoietic cell markers) in hEnMSCs.
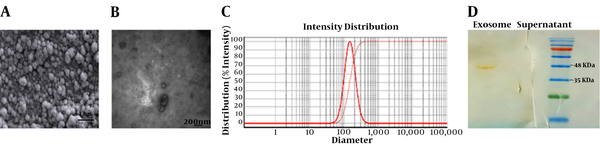
After isolation and characterization of the hEnMSCs (27), the cells were cultured to extract their exosomes. Here, differential ultracentrifugation method was used to isolate hEnMSCs exosomes. Total yield of isolated exosomes was determined nearly 1 mg/mL by Bradford assay. Nature of exosomes isolated from culture supernatants of hEnMSCs was confirmed using several methods. First, the exosomes were subjected to electron microscopic analysis. SEM and TEM analysis indicated round, cup-shaped particles with a diameter ranging 30 to 150 nm (Figure 2A and B). DLS was also performed to verify the vesicle size distribution (Figure 2C). Finally, Western blot technique was also utilized to assess the exosome-specific surface marker CD63. Coomassie blue-stained SDS-PAGE (12% gel) revealed that CD63 was strongly detected in the isolated exosomes (Figure 2D).
4.2. The hEnMSC-Derived Exosomes Uptake by HUVECs
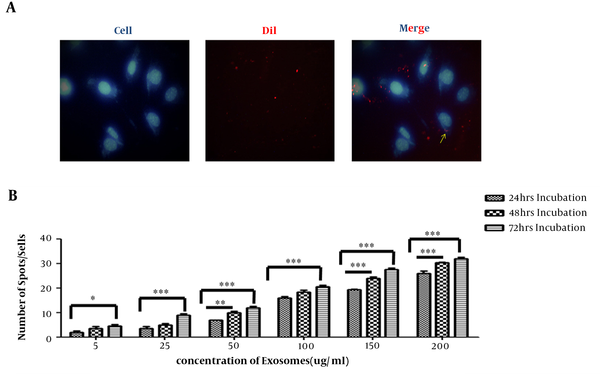
Internalization of hEnMSC-derived exosomes into HUVECs was investigated through labeling exosomes and incubating them with cells. Increasing concentrations of DiI-labeled hEnMSC-derived exosomes were added to HUVECs culture and incubated for 24 hours; uptake of DiI-labeled exosomes by HUVECs was detected using fluorescence microscope at different times after incubation (Figure 3A). A strong correlation was observed between the increasing amount of exosomes as well as the time of incubation and increasing the number exosomes in cells. Exosomes uptake was increased with longer incubation time and increasing exosome concentration (Figure 3B).
Incorporation of hEnMSCs exosomes. A, fluorescent microscope image showing internalization of DiI-labeled hEnMSC-derived exosomes (red fluorescence) in the cytoplasm of the HUVEC cells. HUVEC nuclei were stained with Hoechst 33342 (blue). B, correlation between the increasing amount of exosomes as well as the time of incubation and increasing exosome numbers in cells. Counting the number of red spot/cell showed an increase in the cellular uptake of hEnMSC-derived exosomes by increasing the amount of exosome and the incubation time.
4.3. The hEnMSC-Derived Exosomes Enhance Endothelial Cell Proliferation
To explore whether hEnMSC-derived exosomes can induce cell proliferation, HUVECs were incubated with 0, 50, 100, 150, and 200 µg/mL hEnMSC-derived exosomes and MTT assay was carried out after 24 and 48 hours of treatment. It was observed that the increasing amount of hEnMSC-derived exosomes could lead to increased HUVECs proliferation. It was also observed that a specific dose of the exosomes could lead to more cell growth with longer incubation time (Figure 4A). In addition, the cytotoxic effects of these exosomes were not observed after three days.
In addition, results of qRT-PCR showed that hEnMSC-derived exosomes significantly increased the expression of proliferative indices (Ki-67 and PCNA) in endothelial cells. Although Ki-67 and PCNA genes were overexpressed in HUVECs treated with 25, 50, 100, 150, and 200 µg/mL of hEnMSC-derived exosomes compared to untreated cells, the highest expression level of both genes was observed at 150 and 200 µg/mL of the exosomes (Figure 4B).
4.4. The hEnMSC-Derived Exosomes Promote Migration of HUVECs
Wound scratch assay revealed that migration of HUVECs into the scratched areas increased in the presence of hEnMSC-derived exosomes. Scratch wound healing assay was performed to evaluate endothelial cell migration. Cell covered area in the presence or absence of hEnMSC-derived exosomes (0, 25, 50, 100, 150, and 200 µg/mL of protein) was pictured and analyzed using a microscope every six hours for 24 hours of wounding. The results showed that hEnMSC-derived exosomes enhanced endothelial cell migration; and the cells in presence of the exosomes migrated faster than untreated cells (Figure 4C). It was observed that 150 and 200 µg/mL exosomes could greatly enhance cell migration; therefore, the scratch was completely filled after 12 hours. Untreated as well as cell-free zones incubated with 25 and 50 µg/mL hEnMSC-derived exosomes were not filled after 24 hours of wounding. However, wounded cell monolayer incubated with 100 µg/mL hEnMSC-derived exosomes was completely filled after 24 hours of treatment (Figure 4D).
hEnMSC-derived exosomes enhance endothelial cell proliferation and migration. A, HUVECs were treated with different concentration of hEnMSC-derived exosomes and MTT assay was carried out after 24 and 48 hours of treatment. Absorbance was measured at 570 nm and normalized against untreated cells. MTT data showed hEnMSCs exosomes increased proliferation of treated cells. B, the qRT-PCR results showed that proliferation markers (PCNA and Ki-67) increased in HUVECs treated with hEnMSC-derived exosomes. C and D, scratch wound healing assay data showed that hEnMSC-derived exosomes enhanced HUVECs migration. Exosomes 150 and 200 µg/mL resulted in scratch filling after 12 hours (C), while cell-free zone in the presence of 100 µg/mL exosomes was filled after 24 hours (D). Scratches incubated with 25 and 50 µg/mL exosomes were not filled after 24 hours of wounding (not shown). All data are expressed as mean ± standard deviation from three independent experiments. * P < 0.05, ** P < 0.01, and *** P < 0.005.
4.5. The hEnMSC-Derived Exosomes Promote Endothelial Cell Angiogenesis in vitro
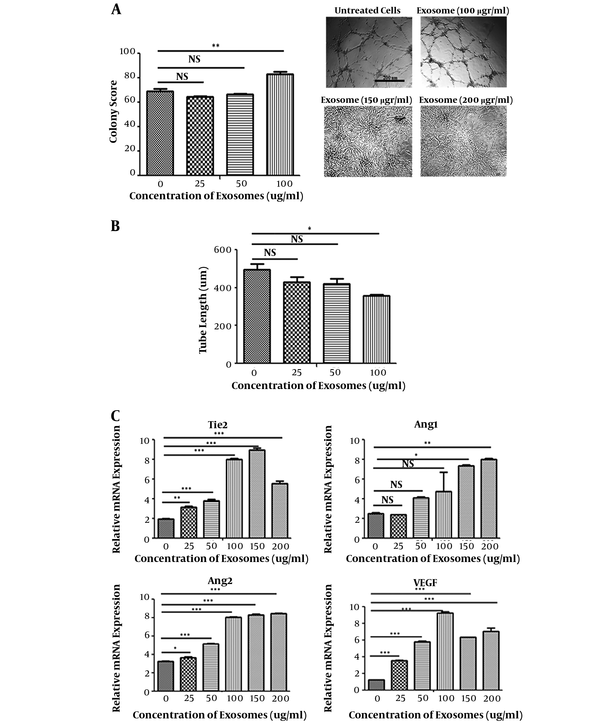
Based on the results of tube formation assay, angiogenesis of HUVECs treated with hEnMSC-derived exosomes significantly increased in a dose dependent manner. There was a significant increase in the colony score of HUVECs treated with 100 µg/mL hEnMSC-derived exosomes, while no significant difference was observed between 25 and 50 µg/mL compared to untreated cells. Angiogenic activity was not observed in HUVECs in the presence of 150 and 200 µg/mL exosomes due to high proliferative activity (Figure 5A). Interestingly, 100 µg/mL of HUVECs treated with hEnMSC-derived exosomes formed higher number of tube forming colonies compared to untreated cells; however, there was a significant increase in tube diameter and a decrease in tube lengths (Figure 5A and B).
In addition, expression levels of angiogenic markers (Tie2, Ang2, Ang1, VEGF) were evaluated using qRT-PCR. Although hEnMSC-derived exosomes led to increased expression of the angiogenesis-related genes, changes in gene expression levels were observed differently. The expression level of VEGF in HUVECs was higher in the presence of 100 µg/mL exosomes compared to other concentrations. The highest expression level of Tie2 was in HUVECs treated with 100 and 150 µg/mL exosomes. Exosome concentrations 150 and 200 µg/mL resulted in a significant increase in the expression level of Ang1; while 100, 150, and 200 µg/mL exosomes almost equally increased the expression level of Ang2 (Figure 5C).
Proangiogenic activity of hEnMSCs exosomes. A, counting the number of tube forming colonies on Matrigel revealed that angiogenic activity of HUVECs significantly increased in presence of 100 µg/mL hEnMSC-derived exosomes, while no significant difference was observed for the treatment of 25 and 50 µg/mL hEnMSC-derived exosomes compared untreated cells. Excessive cell proliferation in the presence of 150 and 200 µg/mL hEnMSC-derived exosomes prevented tube forming activity of HUVECs. B, HUVECs treated with hEnMSC-derived exosomes formed tubes with an increase in the diameter and a reduce in the length of the tubes compared to untreated cells. C, hEnMSC-derived exosomes enhance expression levels of angiogenesis-related genes (Tie2, VEGF, Ang2, and Ang1) in HUVECs.
5. Discussion
Recently, MSCs are the subject of a wide variety of clinical studies including drug/gene delivery (28), immune modulation (29), tissue repair (30), and diagnosis/treatment of cancer (31, 32). There are various tissue sources to isolate these cells; most commonly is BMSCs.
Several studies show that hEnMSCs could enhance angiogenesis (7, 9, 18, 33). Alcayaga-Miranda et al., reported that hEnMSCs exhibited superior angiogenetic properties compared to BMSCs (23) and the beneficial effects of hEnMSCs therapeutic angiogenesis on heart failure and critical limb ischemia are confirmed by several preclinical studies (24-26). However, the underlying mechanisms of the proangiogenic effects of hEnMSCs remain unclear.
The results of the current study suggested that the angiogenic effects of hEnMSCs in vitro can be partly due to hEnMSCs exosomes. Here, hEnMSCs were successfully isolated from endometrial tissue specimens. As shown in Figure 1B, the cells positively expressed MSC markers such as CD90, CD105, and CD146by 98.4%, 76.7%, and 46.5%, respectively. The isolated cells were negative for expression of hematopoietic cell markers such as CD31 (1.97%), CD34 (3.02%), and CD45 (2.11%). Then exosomes were isolated from culture medium of hEnMSCs. Total yield of extracted exosomes was nearly 1 mg/mL. As shown in Figures 2A and C, exosomes isolated from hEnMSCs were heterogeneous in size, with approximate diameter of 30 to 150 nm. Immonobloting data showed that the exosomes were positive for the surface marker protein CD63. In addition, hEnMSC-derived exosomes could incorporate into the cytoplasm of endothelial cells (Figure 3A) where they enhanced proliferation, migration, and proliferative gene expression (Figure 4).
As shown in Figure 3B, image analysis of fluorescence microscope indicated that more exosomes were internalized into endothelial cells by increasing incubation time and concentration of exosomes. Therefore, cell proliferation was investigated in the presence of increasing concentrations of hEnMSC-derived exosomes at different incubation time. It was observed that more cellular uptake of hEnMSC-derived exosomes led to more endothelial cell proliferation and higher expression levels of proliferative genes (PCNA and Ki-67). However, approximately equal cell proliferation and gene expression levels in HUVECs treated with 150 and 200 μg/mL hEnMSC-derived exosomes can be due to high cell confluence and contact inhibition (Figure 4A and B).
The MTT and scratch wound healing data showed that 150 and 200 μg/mL hEnMSC-derived exosomes increased endothelial cell proliferation much more than other concentrations, which led to much faster cell proliferation and migration; hence, the scratches were completely filled after 12 hours of treatment (Figure 4C). After 24 hours of treatment, it was observed that cell wells were filled with cellular debris due to extremely large proliferation of cells and their multi-layered growth.
In addition, the ability of extremely increased cell proliferation in the presence of 150 and 200 μg/mL hEnMSC-derived exosomes resulted in the inhibition of endothelial cell angiogenesis onto Matrigel. Nevertheless, 100 μg/mL hEnMSC-derived exosomes was the optimal concentration that significantly increased endothelial cell proliferation as well as migration and angiogenic activity, as shown in Figures 4D and 5A.
Moreover, hEnMSC-derived exosomes induced tube forming colonies and angiogenic gene expression, suggesting that hEnMSC-derived exosomes can be partly responsible for the angiogenic effects of hEnMSCs.
It was observed that hEnMSC-derived exosomes enhanced endothelial cell angiogenesis in a dose dependent manner. Endothelial cell colony score significantly increased only in the presence of 100 μg/mL hEnMSC-derived exosomes. Interestingly, it was observed that endothelial cells treated with 100 μg/mL hEnMSC-derived exosomes significantly increased the number of tube forming colonies, and also reduced the length of the tubes (Figure 5A and B).
The expression levels of angiogenic genes (Tie2, Ang1, Ang2, and VEGF) increased in endothelial cells treated with hEnMSC-derived exosomes compared to untreated cells (Figure 5C). There is the possibility that the difference in the expression levels of these genes in endothelial cells in the presence of different concentrations of exosomes lead to differences in the angiogenic activity of cells.
Besides, analysis of tube formation images showed that hEnMSC-derived exosomes led to increased tubes branched out from each colony. However, the mechanisms by which hEnMSC-derived exosomes enhance these angiogenic activities of endothelial cells are studied continuously.
The components and hence the effects of exosomes on their recipient cells can vary depending on the tissue source and environmental context of MSCs. Exosomes can carry various molecules and exert their biological effects via transporting these molecules to recipient cells. Here, for the first time, it was demonstrated that in addition to growth and migration, hEnMSC-derived exosomes can enhance angiogenesis of endothelial cells. These results provide the first evidence for the potential of hEnMSC exosomes in wound healing. However, the discovery of the exosome components and exact molecular mechanisms responsible for these stimulatory effects of hEnMSC exosomes are suggested. The study on signaling pathways and key factors involved in angiogenesis after treatment of cells with these exosomes may be helpful.
5.1. Conclusions
In summary, the current study findings suggested that the hEnMSC-derived exosomes were the positive regulator of angiogenesis in a dose dependent manner and might be promising tools to apply in wound healing, and treatment of vascular disease.