1. Background
Herpes simplex encephalitis (HSE) is the most prevalent form of sporadic encephalitis worldwide, which is a potentially fatal infection of the central nervous system (CNS) (1). The incidence of HSE worldwide is one to two cases per 500,000 population per year (2). The development of antiviral therapy has decreased the mortality rate of HSE from 70% to 5% - 15% (3). Herpes simplex encephalitis is a severe, devastating viral infection of the CNS caused by either herpes simplex virus-1 (HSV-1) or HSV‐2, predominantly HSV-1 (3). Also, HSE is an acute focal necrotizing inflammatory process, usually affecting the cortex and underlying white matter of the frontotemporal lobe of the brain (4). In less common situations, the insula, cingulate gyrus, and posterior orbitofrontal lobe of the brain are involved in HSE (5). In rare conditions, the brainstem may be affected, too (1). Approximately half of the HSE patients experience the symptoms of extra-temporal involvement, occasionally even without any temporal abnormalities (5). The combination of cytolytic viral replication and immune-mediated mechanisms can affect the CNS and lead to axonal and glial damage (5).
Generally, HSE is characterized by fever, headache, seizure, altered consciousness, disorientation, behavior or personality changes, and often focal neurological deficits (6). Herpes simplex encephalitis is confirmed definitely through the result of HSV DNA in cerebrospinal fluid (CSF) by the polymerase chain reaction (PCR) assay (7). The clinical guidelines emphasize early treatment with acyclovir that has considerably decreased the mortality rate of HSE to 20%, as well as its morbidity (8). Nonetheless, HSE usually leads to serious complications, poor outcomes, and occasionally permanent neurological sequels and disabilities (4, 5, 9).
Lumbar puncture (LP) is an essential procedure to support the diagnosis and ensure CSF for further analyses (8). Focal EEG abnormalities frequently involving temporal lobes are seen in 75% to 80% of patients with HSE. Common abnormalities include the presence of frontotemporal slowing, temporal sharp or spike activity, and periodic lateralized epileptiform discharges at a rate of 2 to 3 Hz. However, none of these patterns is pathognomic for HSE (10). In the brain MRI of HSE patients, one may see high signal intensity lesions on T2-weighted and FlAIR images involving the medial and inferior temporal lobes with extension into the insula. Also, abnormal findings may be seen in the orbitofrontal gyri and inferomedial frontal lobes (11).
2. Objectives
Due to the high mortality rate and serious complications and disabilities of HSE, recognizing HSE patients is the first vital step to diagnose the disease and start treatment immediately. In this study, we aimed to evaluate the clinical/paraclinical findings and clinical outcomes of patients suspected to HSE.
3. Methods
3.1. Participants
In a descriptive-analytical study, 70 patients suspected to HSE were admitted to the Emergency Department (ED) of Imam Khomeini Hospital, Tehran, Iran, from March 2017 to November 2018. They were prospectively enrolled in the study to be evaluated for clinical/paraclinical findings and clinical outcomes. This research was performed after receiving permission from the Research Council and Ethics Committee of Tehran University of Medical Sciences (TUMS). The authorities of Imam Khomeini Hospital and the ED of the hospital agreed with the study. Patients’ information remained confidential throughout the study. Written informed consent was obtained from the patient's immediate family before enrollment, and the researcher provided explanations about the study procedure.
The inclusion criteria were suspicion to HSE according to clinical signs and symptoms and physical/neurological examinations, administration of intravenous acyclovir by a physician, and no contraindications for LP. The exclusion criteria were the acquired immune deficiency syndrome (AIDS) and young age (less than 14-years-old). Patients with fever and decreased level of consciousness who were suspected to HSE were enrolled in this research.
3.2. Materials
For data collection, we used the demographic and HSE information checklists. The demographic checklist consisted of information such as age, sex, level of consciousness (LOC) based on the Glasgow coma scale (GCS), past medical history, onset and duration of symptoms, the lag between initial symptoms and treatment onset, treatment period, and the lag between clinical symptoms onset and hospital admission. In the HSE checklist, we recorded information such as clinical findings (altered consciousness, seizure, fever, dysarthria, bizarre behavior, personality changes, decreased muscle tone, Babinski reflex, disorientation, and cranial nerve palsy), paraclinical findings (laboratory and imaging investigations), and outcomes (discharge from hospital, survival with neurological sequels, and death).
Bizarre behavior was defined as any abnormal behavior (restlessness, agitation, involuntary, and unintelligible reflexes). Personality changes were determined by any abnormal clinical personality changes such as delusion, hallucination, abusiveness, etc. that the patient did not show previously, and they were due to the neurological sequels of HSE. Also, cranial nerve palsy and other signs and symptoms were evaluated by the researcher using history-taking and physical/neurological examination on admission.
The laboratory tests on blood and CSF samples were performed on admission or during the initial 24 hours after admission. Imaging studies including brain magnetic resonance imaging (MRI) and electroencephalogram (EEG) were performed during hospitalization. Blood tests and CSF analyses were performed with Sysmex XE-2100 (in 2014) and Sysmex XN-9000 (in 2015), respectively. The CSF samples were centrifuged at 1,500 rpm for 5 minutes to remove cells and then aliquoted and stored at -70°C. Herpes simplex encephalitis was confirmed with positive HSV PCR of CSF analysis.
According to the final diagnosis, clinical/paraclinical findings, and medical treatment, the patients were divided into three groups: patients with confirmed HSE, patients with a diagnosis other than HSE, and patients suspected to HSE (with no definitive HSE diagnosis). Each patient was followed up for clinical outcomes until the end of the treatment period in the course of the disease. For example, in the HSE group, the patients were evaluated at the end of the treatment period with acyclovir. In the current study, neurological sequels were defined as any neurological deficits and disabilities following the disease, such as cognitive and memory disturbance, behavioral impairments, disequilibrium, tic disorders, etc. Finally, the clinical/paraclinical findings and clinical outcomes were compared between the three groups.
3.3. Data Analysis
The data were analyzed by the Statistical Package for Social Sciences version 20 (SPSS V. 20.0; SPSS Inc., Chicago, IL, USA) using descriptive statistics (frequency, mean, and standard deviation) and inferential statistics (ANOVA, Kruskal-Wallis H-Test, chi-square test, Student’s t-test, and Mann Whitney test). Statistical significance was defined as a P < 0.05.
4. Results
Seventy eligible patients fulfilled the eligibility criteria. The number of patients in each group was seven in the confirmed HSE group, 40 in the other-diagnosis group, and 23 in the without-diagnosis group, respectively. Seven out of 70 patients had confirmed HSE and entered the confirmed HSE group. The other-diagnosis group included patients with pneumosepsis (n = 10), aspiration pneumonia (n = 10), bacterial meningitis (n = 5), urosepsis (n = 4), neurobrucellosis (n = 2), infective endocarditis (n = 1), phenytoin toxicity (n = 1), autoimmune encephalitis (n = 1), brain abscess (n = 1), opium overdose (n = 1), influenza (n = 1), stroke (n = 1), pulmonary tuberculosis (n = 1), and sepsis following soft tissue infection (n = 1).
Two patients were excluded from the study due to positive HIV tests during research. Two other patients were excluded, one of whom because his family did not allow for LP and the other one because of a contraindication of LP related to the extensive and purulent pressure ulcer on the lumbar area.
All participants were unconscious and febrile on admission. The difference in the mean age was statistically significant between the three groups (P = 0.016) so that the HSE group patients were younger than the other groups. There was a statistically significant difference between the three groups in sex (P = 0.027), and most patients in the HSE group were female. The mean duration of the treatment period was 19.1 ± 6.4 days in the HSE group that was significantly longer rather than that in the other groups (P < 0.001).
Among the clinical characteristics of HSE, only seizure showed a statistically significant difference between the three groups (P = 0.001), and more than half of the HSE patients experienced a seizure. Other clinical manifestations were not significantly different between the three groups (Table 1).
| Medical Diagnosis Variable | Herpes Encephalitis (N = 7) | Other Diagnoses (N = 40) | Without Diagnosis (N = 23) | P Value |
|---|---|---|---|---|
| Age (years) | 39.1 ± 19.2 | 61.9 ± 20.5 | 49.6 ± 17.2 | 0.016 |
| Sex | ||||
| Male | 1 (14.3) | 27 (67.5) | 12 (52.2) | 0.027 |
| Female | 6 (58.7) | 13 (32.5) | 11 (47.8) | |
| Level of consciousness (GCS) | 9 ± 2.3 | 9.2 ± 1.9 | 8.4 ± 2.0 | 0.360 |
| The lag between clinical manifestation onset and treatment onset (days) | 3.4 ± 2.6 | 2.9 ± 2.2 | 3.3 ± 3.2 | 0.940 |
| Treatment period (days) | 19.1 ± 6.4 | 4.8 ± 4.8 | 9.2 ± 5.4 | < 0.001 |
| The lag between clinical manifestation onset and hospital admission (days) | 2.7 ± 2.1 | 2.8 ± 2.0 | 3 ± 3.5 | 0.753 |
| Bizarre behavior | 0.342 | |||
| No | 0 (0) | 2 (5) | 0 (0) | |
| Yes | 7 (100) | 38 (95) | 23 (100) | |
| Personality changes | 0.661 | |||
| No | 2 (28.6) | 13 (32.5) | 5 (21.7) | |
| Yes | 5 (71.4) | 27 (67.5) | 18 (78.3) | |
| Dysarthria | 0.408 | |||
| No | 0 (0) | 4 (10) | 4 (17.4) | |
| Yes | 7 (100) | 36 (90) | 19 (82.6) | |
| Seizure | 0.001 | |||
| No | 2 (28.6) | 36 (90) | 16 (69.4) | |
| Yes | 5 (71.4) | 4 (10) | 7 (30.4) | |
| Decreased muscle tone | 0.395 | |||
| No | 6 (85.7) | 29 (72.5) | 14 (60.9) | |
| Yes | 1 (14.3) | 11 (27.5) | 9 (39.1) | |
| Sensory level | 0.522 | |||
| No | 7 (100) | 26(90) | 22 (95.7) | |
| Yes | 0 (0) | 4 (10) | 1 (4.3) | |
| Babinski reflex | 0.675 | |||
| Downward | 7 (100) | 38 (95) | 21 (91.3) | |
| Upward | 0 (0) | 2 (5) | 2 (8.7) | |
| Cranial nerve palsy | 0.047 | |||
| No | 5 (71.4) | 25 (62.5) | 21 (91.3) | |
| Yes | 2 (28.6) | 15 (37.5) | 2 (8.7) | |
| Altered LOC | ||||
| No | 0 (0) | 0 (0) | 0 (0) | |
| Yes | 7 (100) | 40 (100) | 23 (100) | |
| Fever | ||||
| No | 0 (0) | 0 (0) | 0 (0) | |
| Yes | 7 (100) | 40 (100) | 23 (100) | |
| Disorientation | 0.631 | |||
| No | 1 (14.3) | 4 (10) | 1 (4.3) | |
| Yes | 6 (85.7) | 36 (90) | 22 (95.7) |
aValues are expressed as mean ± SD or No. (%).
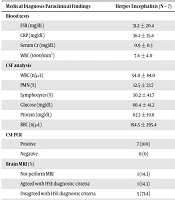
Concerning laboratory blood tests, the mean of erythrocyte sedimentation rate (ESR) was lower in the HSE group (P = 0.034), and the mean of C-reactive protein (CRP) was higher in the other-diagnosis group (P = 0.0231); they were significantly different blood tests between the three groups. No parameter of the CSF analysis showed significant differences between the three groups (Table 2).
The findings of brain MRI were in agreement with the diagnostic criteria of HSE in 71.4% (n = 5) of the HSE patients; the difference between the three groups was statistically significant (P < 0.001. The results of the EEG were not significantly different between the three groups (Table 2).
| Medical Diagnosis Paraclinical Findings | Herpes Encephalitis (N = 7) | Other Diagnoses (N = 40) | Without Diagnosis (N = 23) | P Value |
|---|---|---|---|---|
| Blood tests | ||||
| ESR (mg/dL) | 31.2 ± 20.4 | 44.0 ± 32.8 | 29.2 ± 35.4 | 0.034 |
| CRP (mg/dL) | 36.1 ± 35.6 | 55.3 ± 47.7 | 39.4 ± 39.8 | 0.0231 |
| Serum Cr (mg/dL) | 0.9 ± 0.3 | 1.2 ± 0.4 | 1.1 ± 0.4 | 0.457 |
| WBC (1000/mm3) | 7.6 ± 4.0 | 12.8 ± 7.3 | 10.3 ± 5.3 | 0.046 |
| CSF analysis | ||||
| WBC (n/µL) | 54.0 ± 84.0 | 722.3 ± 3315.8 | 80.5 ± 195.9 | 0.551 |
| PMN (%) | 12.5 ± 23.7 | 24.3 ± 37 | 16.2 ± 28.8 | 0.904 |
| Lymphocytes (%) | 30.2 ± 41.7 | 10.7 ± 21.6 | 18.5 ± 31.3 | 0.642 |
| Glucose (mg/dL) | 80.4 ± 41.2 | 71.6 ± 42.7 | 84.1 ± 44.8 | 0.875 |
| Protein (mg/dL) | 62.3 ± 19.8 | 148.2 ± 450.5 | 76.9 ± 98.2 | 0.570 |
| RBC (n/µL) | 194.5 ± 295.4 | 1011.5 ± 2876.4 | 564.9 ± 1412.9 | 0.527 |
| CSF PCR | < 0.001 | |||
| Positive | 7 (100) | 0 (0) | 0 (0) | |
| Negative | 0 (0) | 40 (100) | 23 (100) | |
| Brain MRI (%) | < 0.001 | |||
| Not perform MRI | 1 (14.3) | 18 (45) | 8 (34.8) | |
| Agreed with HSE diagnostic criteria | 1 (14.3) | 19 (47.5) | 14 (60.9) | |
| Disagreed with HSE diagnostic criteria | 5 (71.4) | 3(7.5) | 1 (4.3) | |
| EEG (%) | 0.591 | |||
| Not perform EEG | 7 (100) | 39 (97.5) | 22 (95.7) | |
| Agreed with HSE diagnostic criteria | 0 (0) | 0 (0) | 1 (4.3) | |
| Disagreed with HSE diagnostic criteria | 0 (0) | 1 (2.5) | 0 (0) |
Concerning clinical outcomes, the differences in outcomes (death or discharge from hospital) and neurological sequels and disabilities after the completion of treatment course were not significant between the three groups (P = 0.646 and P = 0.029, respectively) (Table 3).
| Medical Diagnosis Variable | Herpes Encephalitis (N = 7) | Other Diagnoses (N = 40) | Without Diagnosis (N = 23) | P Value |
|---|---|---|---|---|
| Final outcome | 0.646 | |||
| Death | 2 (28.6) | 7 (17.5) | 6 (26.1) | |
| Discharge | 5 (71.4) | 33 (82.5) | 17 (73.9) | |
| Neurological sequel | 0.029 | |||
| No | 4 (57.1) | 35 (87.5) | 22 (95.7) | |
| Yes | 3 (42.9) | 5 (12.5) | 1 (4.3) |
aValues are expressed as No. (%).
5. Discussion
The results of this study revealed that the mean age was significantly lower in the HSE group than in the other two groups. Mancini showed that the age-specific incidence of HSE was bimodal, with approximately one-third of cases observed in children between three months and 20 years of age and the rest in adults over 60 years encompassing about two-thirds of the patients (1). Also, there was a significant difference in sex between the HSE group and the other two groups, and only one male patient was among the HSE patients.
The rate of seizure was significantly higher in the HSE group than in the other two groups. Therefore, the history of recent seizures can help diagnose HSE. Other clinical findings of history, physical, and neurological examination listed in Table 1 did not show significant differences between the three groups. Therefore, clinical findings did not differentiate HSE from other diagnoses because these findings can mimic the symptoms of other similar diseases. In contrast to our results, Sili et al. concluded that the most common clinical symptom of HSE was altered LOC, which was seen in 100% of the confirmed HSE patients (12). The possible reason may be the enrollment of patients with similar clinical manifestations to HSE and suspected to HSE so that the difference in clinical symptoms between the three groups was justifiable.
The differences in mean ESR and CRP were statistically significant between the HSE group and the other two groups, and the mean of these two blood tests was higher in the other-diagnosis group than in the HSE group. This was possibly related to the higher mean age and the presence of serious infectious diseases such as pneumosepsis, urosepsis, and infectious endocarditis among patients of the other-diagnosis group. Therefore, one should not expect very high levels of ESR and CRP, as well as bacterial infections, in patients with HSE.
The MRI results were significantly different between the HSE group and the other groups and they agreed with the diagnostic criteria of HSE in MRI. This finding can be used as a valuable marker for differentiating HSE from other similar diseases. In the current study, EEG was performed only for two out of 70 participants. It seems to be because of the low sensitivity of this method for HSE diagnosis. In agreement with our finding, Leite et al. indicated that MRI is the test of choice for the diagnosis of encephalitis, but it is not always accessible in the EDs of many hospitals (6). In a similar study conducted by Sili et al., about 95% of patients had MRI findings in favor of HSE characteristics and the treatment period was 19 ± 5 in the HSE group. In the HSE group, 34 (71%) patients experienced neurological sequels, and five (9%) patients died; the difference between the two groups was not significant (12). Also, Sili et al. declared that 38% of HSE patients showed abnormal findings in favor of HSE in brain CT scan and 95% in brain MRI, and 87% of the mentioned cases had temporal lobe involvement (12).
Finally, we evaluated the clinical outcomes of participants. The rate of neurological sequels was higher in the HSE group than in the other groups, which is due to the invasion of the herpes virus to neurons, apoptosis, and tissue damage, leading to neurological sequels. Two patients in the HSE group died. For these patients, there was a seven-day interval between the onset of symptoms and the initiation of treatment with acyclovir. Therefore, it seems that delayed treatment with acyclovir affected the final prognosis of HSE patients.
In the present study, the sampling period was longer than the duration anticipated at the beginning of the research, due to the low prevalence of HSE in the community. Also, the number of patients was lower in the HSE group than in the other two groups, so that the differences in the results were not significant in some of the comparisons. We suggest that future studies evaluate more HSE patients.
5.1. Conclusions
In conclusion, the results of the present study revealed that history-taking and past medical history could be used for the precious differentiation of HSE from other similar diseases that mimic the signs and symptoms of HSE. Among clinical manifestations, the recent seizure was a helpful sign for the diagnosis of HSE. Also, the evidence of temporal or frontal lobe involvement in brain MRI and increased signaling in these lobes are good markers to diagnose HSE. Therefore, we propose to perform brain MRI for patients suspected to HSE as early as possible. In addition, we found that the early use of acyclovir for suspected patients plays a pivotal role in the improvement of the clinical prognosis of HSE and we suggest that physicians prescribe acyclovir as soon as HSE is clinically suspected.