1. Context
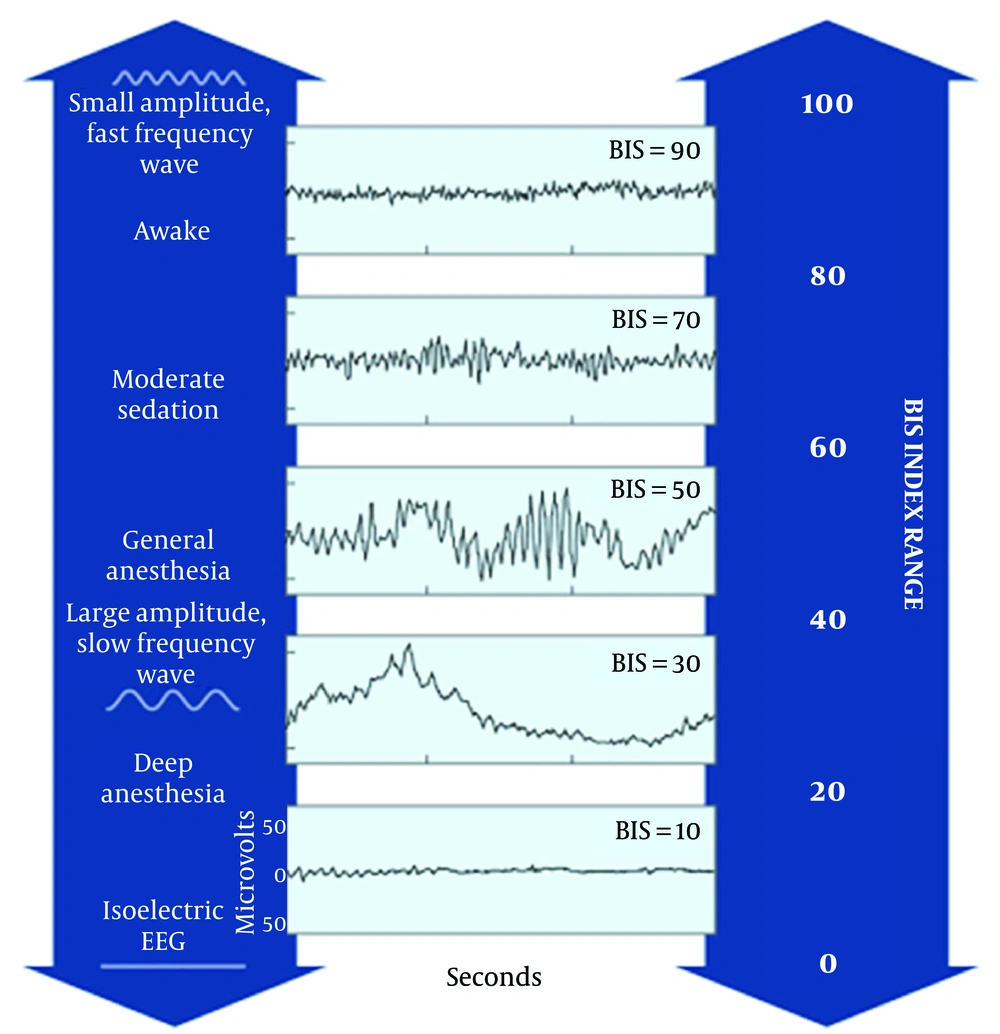
Bispectral analysis was first introduced in 1960 to analyze the EEG waveforms and generate a numerical output which ranges from zero (EEG suppression) to 100 (full consciousness) by merging the attained data with a sophisticated algorithm. Figure 1 Aspect Medical Systems, Inc offered the first commercial BIS in 1993. US Food and Drug Administrative (FDA) approved the BIS monitoring in 1996, as a tool to evaluate and monitor the effect of anesthetics and sedative drugs. Previous studies have shown the BIS index values between 40 and 60 to indicate the appropriate level of general anesthesia (1-3).
Providing adequate and safe analgesia and sedation for patients in intensive care units, particularly for procedures and mechanical ventilation, is an essential component of ICU care. Frequent monitoring of the sedation depth can ensure the achievement and maintenance of the desired level of sedation (4-6).
Ramsay Sedation Scale (RSS), Sedation-Agitation Score (SAS) and Glasgow Coma Scale (GCS) are commonly clinical tools to monitor sedation, agitation, and level of consciousness, respectively in the ICU (7). RSS scales patients into six levels of sedation from restlessness to deep coma and SAS defines 7 levels of agitation and sedation from unarousable to dangerously agitated state. Other clinical/physiological signs such as heart rate, blood pressure, sweating, pupillary size, body movements, eye-opening and tears have also been employed to evaluate the state of sedation and analgesia (8, 9). However, these measures have several limitations and could be influenced by many other factors that may lead to over-sedation or under-sedation. Therefore, the quantitative methods such as direct brain activity monitoring by the EEG signal analysis could be an effective adjunct to monitor the depth of sedation in the ICU (10-14).
2. Application of BIS During General Anesthesia
General anesthesia (GA) consists of three stages: induction, maintenance, and recovery. By applying the BIS monitoring, we can obtain useful information in the course of each stage of anesthesia. During the maintenance phase of GA, the optimum range of the BIS index is between 40 and 60. Maintaining the BIS index in this range reduces the anesthetic drug usage and shortens the emergence time in the PACU.
If the anesthetic agents fail to create or maintain an adequate anesthesia depth, there is significant potential for the patient to regain sensorium during the maintenance phase of anesthesia. The operation recall is one of the most important reasons for grave emotional and physical consequences to the patient; it is one of the most important complaints against anesthesiologists. Therefore, anesthesiologists are determined to minimize the level of consciousness and maintain appropriate anesthesia depth while using the minimum required amounts of anesthetic agents.
A study based on the BIS monitoring with a large sample size (n = 967) showed that the incidence of recall was minimal (0.62%) in GA during surgery. Several other studies have indicated BIS monitoring to reduce the anesthetic drugs and opiates use during general anesthesia (15, 16). In addition, the reduction in the incidence of awakening during anesthesia and the anesthesia recovery time were shown in multiple other studies (17, 18).
Cognitive impairment and delirium are other potential side effects of GA. Studies have indicated a reduction of these complications via BIS during GA (19). Furthermore, Herrero et al. showed BIS monitoring potentials in the early detection of neurological complications in the PACU and consequently improved survival with their prompt management (20).
Despite the BIS monitoring and inappropriately higher doses of the anesthetic drugs administration, wakefulness during surgery has also been reported in the literature. Morse et al. used BIS to monitor sedation levels in patients undergoing oral surgery with anesthesia by midazolam and a midazolam-ketamine combination. They showed that ketamine does not affect the BIS value which remained close to the baseline. They concluded that BIS monitoring was not useful in their study subjects (21). The use of BIS in general anesthesia is listed in Table 1.
| Variables | Effects of BIS on Variable | References | |
|---|---|---|---|
| 1 | Sedative dosage | Reduce the sedative dosage | (15, 16) |
| 2 | Awareness | reduce the incidence of unexpected awareness during surgery | (17, 22-25) |
| 3 | Recovery time | BIS monitoring in craniotomy reduced the anesthetic and narcotic drug dosage and lowered the recovery times | (17, 18) |
| 4 | Postoperative delirium and cognition | BIS-guided anesthesia reduced anesthetic dosage and decreased the risk of postoperative cognitive dysfunction at 3 months after surgery | (19) |
| 5 | Postoperative surveillance | BIS with the assessment of pupils, GCS, CNS, Nu-DESC improved early detection of postoperative neurological complications in the Post-Anesthesia Care Unit (PACU) after elective craniotomies. | (20) |
| 6 | Muscle relaxant | Not studied | - |
3. Application of BIS in the ICU
Inadequate sedation in the ICU may lead to significant patient discomfort and pain while over-sedation increases the risk of ventilator-related events and longer ICU stay. However, the BIS monitoring application in the ICU differs from the operating room, considerably by broader BIS index range and diversity of clinical scenarios observed in the ICU (26, 27).
BIS monitoring could be beneficial for the ICU patients, both clinically and financially. Some studies have shown that administration of adequate sedation and analgesia, guided by BIS index, have significantly decreased both the ICU painful events, medication and overall ICU cost (28, 29). However, other studies in patients receiving sedation in the ICU have shown the BIS index to correlate poorly with the Sedation-Agitation Scale (12, 14, 30).
Unlike the monitoring of consciousness and pain perception by the vital signs, BIS is more sensitive in patients undergoing invasive procedures in the ICU, such as intubation, tracheostomy, chest tube placement bronchoscopy, etc (31, 32). However, BIS readings could be rendered less reliable in clinical situations, such as administration of relatively low-level sedation, minor interventions, administration of neuromuscular blockade (NMB), electrical noise interference and abnormal brain function (33). False elevation and false reduction of the BIS index values could be seen in the accentuated EMG signals and administration of NMBs, respectively.
Due to the complexity of care in the ICU and the variety of conditions that may interfere with reliable BIS readings, the determination of optimal sedation based on this modality alone could result in inappropriate administration of sedative drugs which may result in over-or-under-sedation. In general, higher BIS indices may be more applicable for the ICU patients than the values used in the general anesthesia in the operating room (34).
Gill et al. tried to validate the BIS values in the intubated ICU patients. Sedation was monitored by recording the clinical parameters and the BIS values. They observed that BIS could not predict reliable values for appropriate sedation in the intubated patients (35). Olson et al. studied potential benefits of BIS in the ICU, illustrating that there was a relationship between the comatose state and BIS value (36). Other studies showed a better correlation between the level of sedation and BIS values in the surgical ICU patients than the heterogeneous patient groups in the general ICUs.
On the other hand, Bell et al. found significant correlations between the BIS values and the Ramsay Sedation Scale. They showed the BIS values of 87.2 and 80.9 corresponded to an RSS of 3 and 4, respectively (37-39). Adesanya et al. conducted a study to compare BIS values with the patient state index. The patient state index (PSI) is a clinically-validated measure of the effect of anesthesia and sedation. The PSI is calculated by a high-resolution 4-channel electroencephalograph (EEG) after advanced noise reduction. Results showed a significant correlation in the over-sedation but not under-sedation situations compared with these two modalities (40).
Dou et al. examined the utility of the BIS index for monitoring patients in a coma. They demonstrated that BIS values could correlate well with the prognosis of coma in the ICU (41). Subsequently, other investigators showed the BIS indices to correlate well with the prognosis of patients in coma and to assist with brain death determination in the comatose patients (41-44).
Some applications of BIS in the ICU are summarized in Table 2.
| Variable | Effect of BIS on Variable | References |
|---|---|---|
| Medication dosage | (15, 16, 21, 32, 34, 45-49) | |
| Propofol | Reduction of dosage to obtain desire sedation 21% | |
| Midazolam | Reduction of dosage to obtain desire sedation | |
| Dexmedetomidine | Reduce sedative dosage | |
| Sedation scores | (31, 39, 40, 50) | |
| RSS | BIS correlated with Ramsay | |
| PSI | BIS correlated with PSI | |
| GCS | BIS correlated with GCS | |
| Adverse effect | (12, 25, 31, 40, 51) | |
| Over sedation | Prevent from over sedation | |
| Under sedation | Prevent from under sedation | |
| Awareness | Prevent from unwanted awareness | |
| Depressed cardiac contractility | Decrease | |
| Hypotension | Decrease |
Abbreviations: GCS, Glasgow Coma Scale; PSI, Patient State Index; RSS, Ramsey Sedation Scale
4. Application of BIS in the NCCU
Traumatic brain injury (TBI), subarachnoid hemorrhage (SAH), refractory intracranial hypertension (RICH), cerebral vasospasm, different types of strokes, coma, cerebral hypo-perfusion and ischemic-hypoxic brain injuries and seizures are the main pathologies in the neurocritical care ICUs (51, 52).
Studies on patients with TBI have shown a significant correlation between BIS values and the levels of consciousness. BIS was found to be useful in monitoring the sedation level and the early detection of brain death in TBI patients (38, 43, 51, 53-55). Moghaddam et al. monitored BIS for several days in patients with different brain injuries, such as cerebral contusion, subdural hemorrhage, and SAH. In their study the BIS values were not significantly different after two days of monitoring in different pathologies, they were significantly different in different brain lesions after three days (55).
Some case reports have shown that the BIS indices have high sensitivity and specificity in monitoring the depth of sedation in the SAH patients (56-58). The use of BIS index and other interventions such as intracranial pressure monitoring, long-term mild hypothermia, and oncotic therapy, could assist the management of RICH (59). A study on 89 patients with SAH monitored by the BIS and GCS values showed statistically significant correlations between these two modalities and the level of alertness, BIS (r = 0.723, P < 0.01) and GCS (r = 0.646, P < 0.01). The BIS index increased with an increasing level of alertness. The mean BIS values measured for coma, semi-coma, stupor and drowsiness were; 0.14 ± 0.23, 38.9 ± 18.0, 60.3 ± 14.5, and 73.6 ± 16.5, respectively (42, 43). In another study, BIS values did not correlate well with the SAH induced cerebral vasospasm (42).
The study of BIS values and the ischemic-hypoxic brain injury has had conflicting results. Some have shown the positive correlation of the BIS values to the extent of ischemic-hypoxic brain injury, especially in the frontal region, and demonstrated a sudden drop in these values to be associated with cerebral hypo-perfusion (60-65). Some studies did not support these observations (66).
BIS has also been studied in seizure disorders, and significant alterations have been reported in its values during seizure (67). Based on the frequencies of the ictal waveform, the BIS values can decrease or increase. BIS may be useful in monitoring seizures in patients on NMB where clinical detection of seizures could be difficult (67).
BIS values may be altered by the EMG signal variations, induced by the facial nerve stimulation in a seizure. Thus, such variations in the BIS values may portend seizure activity and prompt the need for immediate intervention (10).
Musialowicz et al. monitored the patients with refractory status epilepticus (RSE) in neurocritical care units by BIS and continuous EEG monitoring. They found that the BIS value of 30 could detect burst suppression and have sensitivity and specificity of 99% and 98% respectively. However, the BIS indices could not recognize the regional epileptic activity (49). Table 3 shows the use of BIS in NCCU as follows:
| Variable | Effect of BIS on Variable | References |
|---|---|---|
| Neuro-surgical critical care | ||
| 1. TBI | Significantly correlated with the level of consciousness. BIS can track levels of sedation in traumatic brain injury patients. Early detection of brain death in patients with severe acute TBI. | (38, 43, 51, 53-55) |
| 2. SAH | BIS showed high sensitivity and specificity in sedated patients after subarachnoid hemorrhage. | (56-58) |
| 3. RICH | BIS combined with intracranial pressure monitoring, long term mild hypothermia, hypertonic therapy helped to control refractory ICH. | (59) |
| 4. Cerebral vasospasm | Was not detectable by BIS. | (42) |
| Neurological critical care | ||
| 1. Coma | BIS is correlated with the prognosis of patients with coma in the ICU. BIS can be used in severely comatose patients as an assessment of brain death. | (41-44) |
| 2. Cerebral hypo-perfusion and ischemic-hypoxic brain injury | Correlated with frontal region hypo-perfusion. Sudden deterioration in BIS values probably indicate cerebral hypo-perfusion. | (60-64) |
| Some studies, however, have not found a significant correlation between BIS values and cerebral hypo-perfusion. | (66) | |
| 3. RSE | Increased BIS values with the onset of seizure. | (68-70) |
Abbreviations: RICH, refractory intracranial hypertension; RSE, refractory statues epilepticus; SAH, subarachnoid hemorrhage; TBI, traumatic brain injury
5. Conclusions
Interpreting the EEG signals could be a challenging task for critical care physicians. Providing numerical values driven from the EEG signals, the BIS index is a relatively easy tool for evaluating the conscious state of patients during anesthesia and recovery. Its application has been studied in other critical care settings, such as monitoring the depth of sedation in the ICU and many other situations in the NCCUs. However, this modality still requires more rigorous investigations before it could find its place in NCCU.