1. Context
Adult multipotent mesenchymal stem cells (MSCs) are one of the most common potential off-the-shelf stem cells that can be isolated from different human tissues (1). They have been examined as interesting choices in regenerative medicine due to their therapeutic potential, including the repair of damaged tissues, antitumor activity, and inflammation suppression (2). Stem cell therapy was first applied for the treatment of various disorders, including muscular dystrophies, myocardial infarction, tumors, kidney damage, and liver damage (3-7). Recent studies have shown that the crucial MSCs therapeutic activities are attributed to paracrine factors such as proteins, cytokines, hormones, and especially exosomes (8, 9).
Exosomes are 30 - 150 nm natural spherical vesicles secreted from direct budding of various cells’ membranes into the extracellular environment and biological fluids, including blood, urine, saliva, and breast milk (10). They play a key role in cell-cell communication through carrying biological compounds (proteins, lipids, RNAs, and other unknown mediators), and they are involved in physiological and pathological processes as essential messengers (11). Although exosomes exert MSCs functions in tissue regeneration, MSC-derived exosomes are nonimmunogenic nanoscale carriers that can protect their cargos (proteins, peptides, DNA, RNA, or other small molecules) from degradation by serum protease or immune system cells (12) and increase drug solubility, stability, and availability in a sustained-release manner (13). Even, they can pass through biological barriers to deliver effective drugs to target tissues; thus, they can participate in delivery systems to develop smarter and safer vehicles. Exosome nanotechnology has provided high compatible, stable, long-time circulating, natural lipid-bilayer nanocarriers to overcome the limitations of nanotechnology in drug delivery systems, such as fast clearance because of the elimination of nanostructured carriers by the reticuloendothelial system (RES), changes of pharmacokinetic behaviors due to protein absorption on their surfaces, and low cellular internalization (14). In this review, we summarize the biogenesis, structure, and content of exosomes. Then, we focus on exosome roles in physiological and pathological processes and their prospects in drug delivery systems. Finally, we discuss the opportunities and obstacles to the clinical translation of therapeutic drug delivery-based exosomes.
2. Extracellular Vesicles Structure, Biogenesis, and Composition
Extracellular vesicles (EVs) were initially represented as quality control systems utilized by cells to remove waste content (15). After years of extensive experimental investigations, EVs were recognized as biologic messengers in cell-cell communication by transferring bioactive components such as functional proteins, nucleic acids, and lipids (16, 17). Lipid bilayer EVs are distinguished as circulating biomarkers of diseases, secreted by many cells (18). Over the last decades, a fast-growing interest has been devoted to EVs’ clinical application, mainly because of their roles in pathological and physiological processes (19). Extracellular vesicles are commonly classified into three subtypes, depending on their sizes, origin cells, formation mechanism, or function. Microvesicles (MVs) or ectosomes result from plasma membrane shedding (100 - 1,000 nm). Exosome isolation faces some challenges because of some similarities between exosomes and some other EVs in their physiological characteristics, size, and density (20). Exosome isolation faces some challenges because of similarities between exosomes and some other EVs in their physiological characteristics, size, and density (21). The crucial difference between different EVs types is thought to be their biogenesis that, in turn, controls their content and function. Other types of EVs are produced by cell membrane budding, while exosomes result from endosomal inward budding into MVBs. From there, some multivesicular bodies are entered into the lysosomal compartment for degradation, whereas other forms, called intraluminal vesicles (ILVs), are released outside the mother cell as exosomes (22). During exosome formation, origin cell information is packed into exosomes, which then can be applied to manipulate target cell fate (23). However, because of simple diffusion, MVs cargos are close to their parental cells in composition. Exosome cargo loading mechanism management is well regulated, although its mechanism is not fully understood.
The endosomal-sorting complex and related proteins (Clathrin, TSG101, and ALIX) required for transport (ESCRT)- dependent and also ESCRT-independent pathways participate in ubiquitinated proteins cargo sorting into exosomes. The formation of exosomes is also controlled by Syndecan heparin sulfate proteoglycans and syntenin (17, 24). In addition, CD9, CD63, CD81, and CD82 tetraspanin proteins arrange the oligomeric complex and are associated with MHC complexes and integrin molecules involved in exosome biogenesis of different cell types (25). Besides, Rab small GTPases proteins are needed for exosome formation; for example, early and late endosome shaping requires Rab5 (26). Moreover, Rab7 is responsible for cargo delivery regulation from MVBs to the lysosome and exosome release after MVBs fusion to cell membrane modulated by Rab35, Rab27a, and Rab27b (27). Reported data have shown that the hnRNPA2B1 protein is required for miRNAs sorting to exosomes (28). The exosome protein content is determined by their origin cells including proteins associated with biogenesis (clathrin, TSG101, and ALIX), GTPases, flotillins, and annexins, which are involved in cellular transport, fusion, and protein CD markers of exosome characterization (CD9, CD63, and CD81) (29). Moreover, heat shock proteins (Hsp70 and Hsp90) related to antigen presentation into MHC molecules are found in exosomes (30). Furthermore, the exosome encompasses nucleic acid components, including microRNAs, mRNAs, tRNA, lncRNA, and viral RNA (31). These natural nanocarriers affect the recipient cells’ transcriptional process and exert multiple functions such as signal transduction exchange and development of organs’ physiological and pathological functions (32). Lipid exosomes contain cholesterols, ceramides, and sphingolipids and show variable distributions of parental cells’ membrane lipids (33). Various mechanisms involved in exosome internalization into recipient cells comprise clathrin-dependent and clathrin-independent endocytosis, as pinocytosis, phagocytosis, micropinocytosis, caveolae, and lipid raft-mediated endocytosis (34). In contrast, they can fuse to recipient cell membranes directly to secret their various biologic components into the targeted cytoplasm (35).
2.1. Exosome Identification and Characterization
To accelerate the investigation of these nanoscale carriers, it is necessary to isolate exosomes from a wide variety of cell culture supernatant components. To study the quality of harvested exosomes, different visualized and non-visualize techniques have been used to explore the exosomes’ size and size distribution (dynamic light scattering and nano tracking analysis) morphology and topography (SEM, TEM, and AFM electron microscopy), and quantity (BCA and Bradford assay), as well as to examine their biological compositions (Western Blot, Flow Cytometry, and ELISA methods) (36). Several exosome isolation techniques are currently utilized to separate and purify exosomes from supernatants of cell culture and biological fluids depending on physical, chemical, or biological properties such as the size, shape, density, molecular weight, surface charge, and special protein markers or exosome CD markers (CD63, CD9, CD81, TSG101, Alix, and etc.) (37). These methods include ultracentrifugation, density-gradient precipitation, ultrafiltration, size exclusion chromatography, and microfluidic-based isolation methods (Table 1). The gold standard method for exosome isolation is Ultracentrifugation. This method starts with some differential centrifugation steps to eliminate dead cell and cell debris, then continue with ultracentrifugation step to remove microvesicles, and some other protein contamination at 120,000 g for 120 min). Today, some commercial exosome isolation kits are applied to purify exosomes because of their advantages such as fast and sensitive isolation of high-yield exosomes from a low volume of cell culture or other biologic fluids (urine and blood). Each isolation technique has advantages and disadvantages that should be considered in terms of being reproducible, specific, and feasible (38).
| Exosome Isolation Technique | UC | SEC | UF | Immunoaffinity | Precipitation | Microfluidics |
|---|---|---|---|---|---|---|
| Exosome Isolation Method | Density-based method | Size-based method | Size-based method | Activity-based method | Activity-based method | Novel-hopeful method |
| Principles of isolation | Using multi-differential centrifugation to isolate exosomes from a heterogeneous population of different biomolecules based on size, shape, and density | Exosome harvesting by size difference through pores | Exosome harvesting by size difference through pores | Exosome capture due to the specific interaction of exosomal surface protein markers (CD63 and CD9) to fixed antibodies | Exosome solubility or dispersibility alteration by synthetic polymers application | Miniaturized equipment to isolate exosomes based on a combination of conventional methods (size, density, and immunoaffinity) |
| Advantages | Cost-effective, easy to use, large-volume samples | Reproducible, feasible, and high purity exosome isolation that maintains exosome functions | Inexpensive instruments and a rapid isolation method with high-purity | Specific isolation of exosomes and excellent purity | Easy to use, short-time process for large-volume samples | Consumption of a low amount of sample, high purity, and cost-effectiveness |
| Disadvantages | Long-time process, low reproducibility, and purity, high-speed ultracentrifugation step damaged naïve exosome morphology | Long-time process with low recovery to large scale production | Loss of the final exosome yield and exosome aggregation due to filtration forces and trapping exosomes in the pores | High-priced reagents, working just for cell-free initial samples, low yield, immune antigen epitope masking because of non-specific antibody binding | The presence of non-exosomal contaminations such as proteins or polymeric molecules at the end of the process | Lack of standard protocols for isolation and confirmation methods, the need for high technical knowledge and skill |
Abbreviations: SEC, Size Exclusion Chromatography; UC, Ultracentrifugation; UF, Ultrafiltration.
3. Therapeutic and Clinical Application of MSC-Derived Exosomes
Mesenchymal stem cells are one of the best candidates for regenerative medicine due to their differentiation potential to produce various cells by the reconstruction of damaged tissues or cells. This type of stem cell is broadly used in clinical trials, although most research has shown that exosomes play a key role in MSCs’ functions (39). Mesenchymal stem cell-derived exosomes exert their therapeutic function through nucleic acids (DNA, RNA, Lon non-coding RNA, and miRNA) and protein components that provide multi-signals through transferring them to target tissues and cells (40). It is reported that the conditioning medium of MSCs can help cardiac regeneration (41), treatment of immune disorders (42), and protection of the kidney and liver from failure damages (42-44). The conditioning medium of MSCs culture is full of exosomes that a famous candidate for MSCs’ therapeutic roles. Many investigations demonstrated that these nanostructure particles exert diverse useful curative effects such as the repair of various tissues, wound healing, anticancer activity, and immunosuppression activity. The published data from Bruno et al. (45) in 2009, clarified acute tubular injury improvement after treating with EVs derived from MSCs. Similar data published by He et al. (46) established the renal protection function of BMMSCs exosomes against murine kidney damage by the reduction of uric acid, creatinine, fibrosis, and immune cell infiltration. In another study, EVs isolated from MSCs enhanced cardiac recovery after ischemia-reperfusion injuries by the Akt/PI3 pathway, GSK3β phosphorylation, and oxidative stress reduction (47). Besides, the potential of MSC-derived exosomes was confirmed in treating liver failure induced by carbon tetrachloride and skin epithelium regeneration in the murine burn model (4, 48, 49). In general, these data show various signaling pathways of exosomal therapeutic effects that are novel hopeful options to treat diseases related to acute injuries and regenerate damaged organs, tissues, and cells. However, extensive pre-clinical studies are still necessary to discover the mechanism of exosome action (48). The similar effects of different exosomes purified from various cell types to their parental cells have been identified in numerous disease models. The advantages of MSC-derived exosomes include the lack of immunogenic problems and immune rejection in the host and the ability of transcytosis through BBB to deliver bio-components to the brain tissue parenchyma (50, 51).
3.1. Exosomes as Nanocarriers
There is a growing interest in the application of exosomes as bio-nanostructures in the delivery system because of their numerous advantages compared to other synthetic nanocarriers such as liposomes. Exosomes have natural nanosized lipid bilayer structures that make them safe and more biocompatible vehicles with low immunogenicity. Furthermore, these vesicles derived from biological fluids or cell culture media can circulate in body fluids and transfer their cargos to neighbor cells and cells at a long distance from origin cells through fusion to plasm membranes of destined cells or phagocytosis; thus, they are the best long half-life carrier system due to their delivery nature. Exosomes are more stable in the gastrointestinal tract and other body fluids without digestive degradation in the stomach or intestine (52). They possess targeted delivery to transport loaded components to target tissue and put them into recipient cells. They are also able to be encapsulated with diverse cargoes because of their capacity to maintain small molecules, miRNA, siRNA, DNA, drugs, and proteins. Exosomes uniquely own therapeutic potential to treat disorders such as different cancers and neurodegenerative diseases and regenerate tissue and cell injuries (53).
3.2. Exosome Loading Methods
The generation of exosomes as bio-nanoscale drug delivery vehicles depends mainly on effective drug encapsulation by suitable drug loading strategies. Up to now, researchers have applied several feasible methods, which are similar to liposome loading strategies, to load desired drugs into exosomes such as simple incubation, incubation with detergents, sonication, electroporation, freeze/thaw cycles, and exosome engineering to improve loading efficacy, e.g., by membrane modification of exosomes. However, some limitations related to unknown issues such as biology, composition, and formation of exosomes and insufficient investigations and development instruments hinder improving exosome loading strategies (54, 55). The key strategies for exosome loading consist of in vitro and in vivo loading methods (56). In vitro drug loading methods introduce a strategy to load both hydrophilic and lipophilic drugs into isolated and purified exosomes. In this way, drugs are entrapped in exosomes by simple incubation that has been employed for hydrophobic molecules such as curcumin, PTX, and Dox encapsulation. The active loading of hydrophilic molecules (nucleic acids and siRNA), which cannot pass through exosome membranes by stimuli (transient disruption of the exosome membrane by chemical or physical stimuli), has been introduced to elevate the amount of drug loading (57). In vitro loading needs exosome isolation methods that provide high-yield purified intact exosomes without any non-exosome contamination. Exosome aggregation or non-exosome contamination is an important barrier to estimate exosome quantification for route administration (58). Another exosome loading approach is drug inserting during exosome formation, called in vivo loading, which has been tried to load cargos such as heavy RNA (mRNA) by using natural mechanisms of xenobiotic/cytotoxic secretion (59). Loading optimization in this approach can be achieved by long-time incubation of drugs and cells to increase the incorporation of hydrophobic drugs into the cells’ cytosol without drug entrapment by lysosomal degradation pathways. Various techniques have been used to harvest loaded exosomes, including starvation, chemical/physical stimuli, oxygen deprivation, and UV application. However, to achieve high loading efficiency without toxicity for origin cells in this method, the exact mechanism related to exosome biogenesis and intracellular pathways should be cleared (60-63).
3.3. Exosomes as Emerging Candidates for Targeted Delivery of Anticancer Drugs
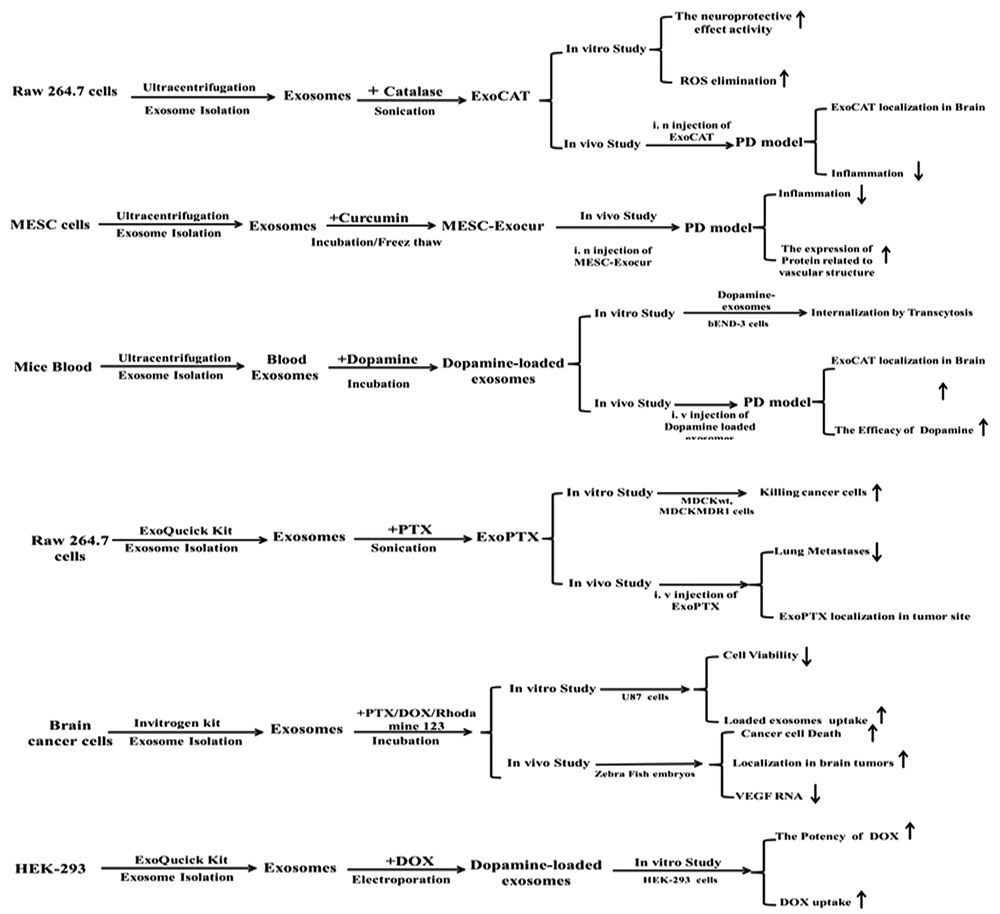
Over the last decades, nanotechnology has been a progressing area in cancer treatment and has provided advantages compared to conventional drug consumption, including decreased systemic toxicity and increased bioavailability, solubility, and half-life of loaded drugs; this has amplified the effects of EPR and the accumulation of drugs in cancer cells to kill cancerous cells (64). Although nanocarriers show beneficial advantages for clinical applications, their use faces many limitations, such as low biocompatibility, internalization, and rapid clearance by phagocytosis. Thus, scientists have started huge efforts to find novel biocompatible nanocarriers. The development of exosome delivery system-based exosomes has established new alternative insight to treat cancer cells because they are highly stable, nanoscale, biocompatible particles that can be loaded with different kinds of therapeutic cargos to deliver cargos and release their content (small molecules, nucleic acids, drugs, or proteins) to tumor cells (65). Chemotherapeutic agents can be successfully encapsulated into exosomes such as paclitaxel, doxorubicin, and hydrophobic phytopolylphenolcurcumin (Table 2) (66). Exosome encapsulation can enhance the efficacy, solubility, and uptake of loaded drugs (67, 68). The targeting capacity of exosomes is 10-fold higher than that of liposomes with the same size because of specific receptor-mediated endocytosis (69). In a study, cytotoxic effects of PTX encapsulated into RAW 264.4 macrophages were explored for overcoming MDR in breast cancer cells. Multi drug resistance (MDR) MDR is a major clinical problem of cancer treatment, and exosomes may be effective nano-drug delivery vehicles to defeat this problem. Kim et al. (70) showed that Exo-loaded PTX was a stable nanoformulation accumulated in cancer cells (examined by confocal fluorescence microscopy after Exo-PTX application in the murine pulmonary metastatic model) and showed more cytotoxic effects compared to free drugs in drug-resistant cells. The Yang et al. (71) investigated bEND.3-derived exosomes loaded with PTX and Dox for brain tumor treatment in the Danio Rorio model. The free Dox and PTX drugs cannot pass through BBB, and thus, their clinical applications are limited for brain tumors. However, the flow cytometry analysis showed the high fluorescent intensity of Rhodamin-labeled exosomes. The cytotoxic effects of loaded exosomes may increase due to the better uptake of loaded exosomes derived from bEND-3 cells into U87 glioblastoma cells (71). In 2019, the HEK293 exosome was encapsulated by Dox to examine the in vitro efficacy of the loaded drug. The uptake (into HEK-293) and cytotoxicity effects of Exo-Dox were higher than those of free Dox and Dox loaded into liposomes (72). These results are similar to the findings of curcumin encapsulation into the exosome to facilitate curcumin insertion to desired cells for improving therapeutic potency. A phase I clinical trial was designed to test the pharmacokinetic and pharmacodynamic properties of exosome-encapsulated curcumin (NCT01294072). However, the loading of small molecules into the exosome may increase the route of drug internalization and lead to the best efficacy of therapeutic molecules in targeted cells.
| Donor Cell for Exosome Isolation | Cargo | Loading Method | Characterization Method | Stability | Release | Function | Reference |
|---|---|---|---|---|---|---|---|
| U-87 MG bEND.3 PFSK-1 A-172 | PTX/DOX Rhodamine 123 | Incubation | CD63, CD9, and CD81 expression | Not reported | Not reported | In vitro: high cellular uptake through receptor mediated-endocytosis; increased cytotoxicity effects. In vivo study: passing through BBB, killing brain cancer cells effectively | (71) |
| Raw 264.7 macrophages | PX | Electroporation | DLS, NTA, Alnix, and TSG101 expression | One-month stability (4°C, RT, 37°C) | Burst release within the first three hours | Elevated toxicity of more than 50 times; Localization of exo-PTX in pulmonary metastases | (70) |
| HEK293 cell line | DOX | Electroporation | NTA, BCA, Alix, CD63, and TSG101 expression | Not reported | Not reported | Fast cellular uptake, improved drug potency after encapsulated into exosome, no cardiac side effects | (72) |
| Raw 264.7 macrophages | Catalase | Sonication | NTA and Bradford | Lyophilized ExoCAT production, one-week stability at 4°C | Sustain release | Significant neuroprotective effects | (73) |
| Blood | Dopamine | Saturated solution incubation | CD63, CD9, and CD81 expression | Increased brain distribution > 15 folds | Slow release | Crossing BBB by transferrin-TfR; without any modifications on exosome | (74) |
| Raw 264.7 macrophages | Curcumin | Incubation | NTA and TSG101 expression | More solubility and bioavailability | Not reported | Inflammation reduction; improvement of vasculature structure | (75) |
3.4. Brain Targeting by Drug Delivery System-Based Exosomes
Neurodegenerative disorders and brain cancers are critical health issues worldwide. The blood-brain barrier (BBB) forms a tight barrier to protect the CNS against drug delivery systems. Just highly hydrophobic molecules with low weight (< 500 Da) can pass through the BBB. It has been supposed that 2% of all molecules can reach the brain tissue by overcoming the BBB. On the other hand, glycoprotein-p that belongs to the ATP-binding cassette (ABC) gene family is the most important element of BBB, which removes large molecules out of the brain endothelium (76). Using nanotechnology is a promising strategy to penetrate the CNS and transfer the therapeutic molecules. Lipid nanoparticles such as liposomes, SLN, and micelle-based lipids are potent carriers for the delivery of drugs in brain disorders. The best nanoparticles for brain targeting are biodegradable, nanostructured components that can cross the BBB and secrete curative biomolecules adequately without making cytotoxicity for normal brain cells (77, 78). However, synthetic nanoparticles with fast elimination by the immune system through the mononuclear phagocyte system (MPS) restrict their targeted administration. Today, exosomes have been introduced as promising brain-targeting vehicles because of their size, plasma membrane-like structure, and the ability to be loaded with various cargos without cytotoxic effects (79). Yuan et al. (80) study in 2017 emphasized exosomes as carriers for small molecules (drugs, RNA, and proteins) to the brain. For the first time, they showed that there is no need for the manipulation of exosomes to pierce the BBB. They found that naïve macrophage-derived exosomes expressed LFA-1 (integrin), which is the ligand of endothelial transmembrane protein ICAM-1. Moreover, the LFA-1-ICAM-1 interaction related to macrophage-exosome internalization into brain endothelial cells and inflammation conditions stimulated ICAM-1 overexpression, resulting in the increase of Mϕ exosome uptake. In vivo intravenous (IV) injection of the Mϕ-derived exosome loaded with brain derived neurotrophic factor (BDNF) can penetrate and accumulate in neural tissues under inflammation stress more than in normal neural cells. Therefore, isolated exosomes from macrophage donor cells can carry therapeutic drugs for brain disorder treatment (80). Haney et al. (73) fabricated exoCAT to treat Parkinson’s disease by effective antioxidant effects of catalase. An in vitro study showed that neuronal cells received a high amount of exoCAT, and when it was administrated intranasally in the PD model, significant neuroprotective effects were detected. Overall, blood monocytes might be good sources to isolate exosomes for loading curative molecules such as catalase to treat inflammatory damage of the CNS (73). In 2008, Chinese researchers developed a nanoformulation of dopamine encapsulated into exosomes harvested from the Kunming mice orbit venous plexus blood for brain targeting in Parkinson’s disease. Loaded exosomes could transfer dopamine to damaged cells of the striatum and substantia nigra areas, and its neuronal distribution was higher than that of free dopamine (> 15 folds) due to the receptor-mediated uptake by transferrin and transferrin receptor (TfR). The safe dopamine-loaded exosome showed powerful therapeutic effects to decrease brain inflammation (74). In another experimental study, exosomes derived from mouse embryonic derived were loaded with curcumin to promote angiogenesis in the mouse model of ischemia-reperfusion (IR). Injected MESC-exocur after seven days decreased the volume of infarct, edema, inflammation, and GFAP expression in astrocyte while it increased NeuN-positive cells. In addition, exosome congaing curcumin improved the expression of claudin-5, occluding, and VE-cadherin to restore the vasculature system in damaged brain tissue (Table 2) (75). Taken together, these studies showed that the exosome could penetrate the brain through the BBB and overcome this important obstacle in neurodegenerative and brain tumor treatment.
3.5. MSCs as Ideal Stem Cell Candidates to Produce Exosomes for Drug Delivery
One of the crucial issues in the drug delivery-based exosome strategy is the choice of exosome donor cells. It is suggested that MSCs are ideal exosome producing candidates in drug delivery approaches. In previous clinical and experimental studies, DC-derived exosomes were exploited as antigen-presenting biomolecules to improve lymphocyte T activation and proliferation (81, 82). However, the immunogenicity of DC-derived exosomes is a major hurdle in their therapeutic application; thus, a perfect exosome producer should be less immunogenic and have the ability to form exosomes on a large scale (83). The exosome large scale production requires a controlled standard process to isolate and purify exosomes from long-term cell cultures. The yield of exosome per cell is an important factor affecting the final quantity and clinical quality of exosomes for drug delivery strategies (84). The production of clinical-grade exosomes through the long-term primary cell culture of MSCs is not a feasible, reproducible approach, and their prolonged cell culture needs the repeated isolation of stem cells from donor tissues; this makes it a costly and time-consuming method with batch-to-batch variations. Thus, researchers need to increase MSCs’ expansion capacity by some manipulation in cell culture conditions (pH, O2, nutrients, Ca+2, endotoxin, and statins) or donor cells (immortalization of MSCs through c-myc transgene overexpression) (85). Ultimately, using filtration, extrusion, and dynamic cell culture methods would improve exosome yields (86). Numerous studies have determined the immunosuppressive and immunomodulatory ability of MSC-derived exosomes as nanocarriers to enhance the protection and bioavailability of loaded cargos (83). Therefore, MSCs should be cultured from different tissue sources, expanded, and then loaded to produce effective therapeutic loaded carriers for drug delivery. A summary of performed studies is shown in Figure 1.
3.6. Challenges to Drug Delivery Application of Exosomes in Clinic
Recently, the FDA has approved 50 nanoparticles with clinical application, e.g., cancer treatment, but clinical administration of exosomes is still maturing (87). Despite the promising advance in the use of exosomes, including DC or MSCsderived exosomes as nano-drug delivery vehicles, there are some considerations for exosome translation from bench to clinical application (88). The development of a functional strategy to increase drug encapsulation ability for strong targeting and cost-effective large-scale production of exosomes is the most important consideration. Understanding the cellular mechanisms of the crossing of exosomes through biologic barriers (endocytosis or receptor-mediated pathways) is also critical for the therapeutic use of exosomes in the drug delivery field. A promising study reported the improved capacity of exosomes to target the brain with RVG peptides. rabies virus glycoprotein (RVG). is a rabies virus glycoprotein peptide that binds to the receptor of acetylcholine on neurological cells (89). On the other hand, exosome components should be analyzed to clarify their genomics, proteomics, and lipid components to eliminate some concerns related to the spread of disorders by exosomes (90). Finally, the therapeutic utilization of exosomes is arrested by the lack of sufficient clinical instruments for up-scale exosome production, isolation, and purification. The ultracentrifugation method provides a heterogeneous population of exosomes that may be contaminated with other components (proteins and unbounded cargos). Thus, the establishment of nanotechnology methods is necessary to prepare the scalable clinical-grade exosomes through standard, reproducible, and feasible protocols for donor cell isolation, purification, and characterization (91).
3.7. Conclusion and Future Perspectives
Recently, many researchers have focused on MSC-derived exosomes to employ their therapeutic and clinical potential in treating injuries and disorders. The most important consideration is the high-yield exosome isolation with sufficient purity and slow clearance that might be manipulated to target desired tissues and cells effectively. The ideal features of MSC-derived exosomes (non-immunogenic, safe, natural, nanosized biomolecules with the ability to pass natural barriers in the body) introduce them as perfect nanocarriers in the next-generation drug delivery system. Moreover, easy and low-cost MSCs culture, which does not make ethical problems, can be regarded as an accepted stem cell production method for large-scale harvesting of exosomes. However, exosome research is a young field and it is critical to explore many aspects of isolated naïve and loaded exosomes including their safety, efficacy, content, composition, real characteristics (shape, size, charge, and specific biologic protein markers), immune responses, and even loading methods. The large scale production of these bio-nanocarriers needs appropriate standard protocols for harvesting and identification of exosomes to translate these biologic carriers from laboratory to clinic.