1. Background
Stroke, as a cardiovascular condition, is a major cause of death and accounts for a large proportion of disability-adjusted life years (DALYs) lost (1-5). The direct and indirect costs of stroke in 2009 have shown to be about $68.9 billion worldwide. Studies have indicated that the global burden of stroke has increased, especially in low and middle-income countries. Ischemic stroke is caused by intracranial thrombosis or extracranial embolism, which respectively arises from atherosclerosis and extracranial arteries or myocardium (6). Although intravenous thrombolysis is the only approved therapeutic method for acute ischemic stroke, there are some barriers, such as prolonged hospital stay for the implementation of this therapy, especially in the developing world (7, 8). The risk of stroke can be identified using some techniques, such as early cardiac evaluation using echocardiography in the emergency rooms (9-12). It has shown that stroke complications can be improved, and also appropriate therapeutic methods can reduce stroke-related mortality or disability (13-16).
Blood biomarkers associated with acute ischemic stroke might improve the prediction of stroke outcomes, which is helpful for early decision-making (17, 18). Bilirubin, as a major intravascular product of heme catabolism, has been found to have biological actions, including anti‐inflammatory, antioxidant, immunomodulatory, cytoprotective, and neuroprotective activities (19, 20). There is a relationship between serum bilirubin levels and the risk of metabolic disorders, such as cardiovascular diseases, type 2 diabetes, metabolic syndrome, hypertension, and obesity (21). Although the high level of serum bilirubin has shown with some positive impacts in the treatment of diseases caused by oxidative stress, these impacts in the acute ischemic stroke are less known (22).
2. Objectives
Considering the numerous studies available, as well as the lack of a relevant systematic review, this study was conducted to evaluate the predictive value of bilirubin levels in ischemic stroke patients.
3. Methods
3.1. Data Sources and Search strategy
We systematically reviewed the literature published in PubMed, Web of Science, Scopus, Embase, ProQuest, and The Cochrane Library with no time limit until October 2018. The search strategy included a combination of Mesh and free keywords, such as Bilirubin, Stroke, Cerebral Ischemia, Brain Ischemia, and Brain Vascular Accident. The used strategies to search databases are provided in the supplementary file, Appendices1 to 6.
3.2. Inclusion and Exclusion Criteria for Patients in the Included Studies
Inclusion criteria for patients were acute ischemic stroke, acute intracerebral hemorrhagic stroke, an acute attack of stroke, ischemic stroke, acute ischemic stroke (AIS) patients admitted to the department of neurology, AIS patients and transient ischemic attack (TIA) patients, and stroke patients.
On the other hand, the exclusion criteria for patients were as follows: liver diseases, congestive heart failure, hematological disease, traumatic hemorrhage, sub-arachnoidal hemorrhage, brain tumor, abnormal platelet count, vascular anomaly, prolonged prothrombin time or prolonged activated partial thromboplastin time, pregnancy, habitual smoking, history of alcohol abuse, intravenous drug abuse, use of antioxidant supplements, hypertension, pulmonary disease and coronary artery disease, diabetes mellitus, hepatic disease, renal disease or renal failure, inflammatory gastrointestinal disease, recurrent stroke, and aspiration pneumonia, severe medical or psychiatric illness, serum creatinine of > 150 µmol/L, gout, use of antioxidant vitamins, previous history of stroke, missing serum total bilirubin level, missing data for a key covariate, established history of renal, hepatic, or cholecystic disease and tumor, pre-stroke impairment, infections, or inflammatory diseases.
3.3. Study Selection
Following the search, all identified citations were collated and uploaded into EndNote, and the duplicates were removed. Then, titles and abstracts were screened by two independent reviewers who assessed the inclusion criteria. Studies meeting the inclusion criteria were retrieved for their full-texts. The full-text of selected studies was assessed in detail based on the inclusion criteria. Full-text studies that did not meet the inclusion criteria were excluded. Included studies were subjected to a critical appraisal. The disagreements between the two reviewers were resolved through discussion or by the assistance of a third reviewer. The results of the search are provided in the final report and presented in a PRISMA flow diagram.
3.4. Assessment of the Methodological Quality
Selected studies were critically appraised for methodological quality by two independent reviewers using the standardized critical appraisal tools. In case of disagreements, discussion, or negotiation with a third reviewer was considered. Following a critical evaluation, studies that did not meet a predetermined threshold for quality were excluded. Studies were excluded based on the degree of bias in the study. If a study was not accompanied by more than two items of the checklists, it was excluded.
3.5. Data Extraction
Data extraction was done using the standardized data extraction tool by two independent reviewers and included surname of the first author, publication year, study design, biomarker, age, sample size, total bilirubin level in cases and control group, stroke severity (NIHSS) neurological outcomes, the correlation between stroke severity and bilirubin, adjusted odds ratio (Tbil-NIHSS), and non-adjusted adds ratio (Tbil-NIHSS). The disagreement between the reviewers in this step was also resolved through discussion or arbitration by a third reviewer. The authors were contacted when any of the data were missing, or additional data were required.
4. Results
4.1. Study Selection
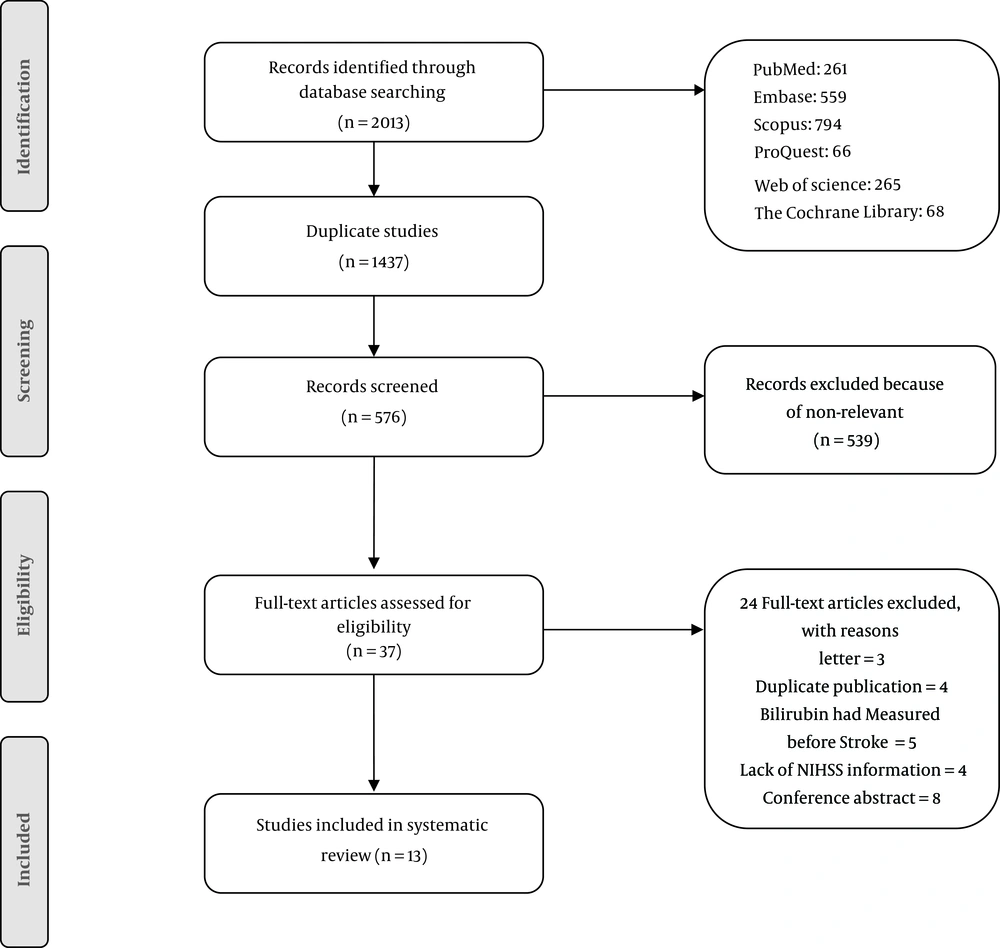
We initially identified 2013 records using the search strategy, of which 576 articles remained after the removal of duplications. Also, 539 articles did not meet the inclusion criteria and were removed after screening for eligibility by reading the titles and abstracts. Thirty-seven full-texts were assessed further by the exclusion of another 24 papers. Finally, a total of 13 studies were included in this systematic review. Figure 1 shows the details of the study selection process. Tables 1 and 2 indicate the risk of bias in the studies.
| Author | Cross-Sectional Studies | |||||||
|---|---|---|---|---|---|---|---|---|
| The Inclusion Criteria in the Sample Were Clearly Defined | The Study Subjects and the Setting Were Described in Detail | The Exposure Was Measured in a Valid and Reliable Manner | The Objective and Standard Criteria Were Used for the Measurement of the Condition | The Confounding Factors Were Identified | The Strategies to Deal with Confounding Factors Were Stated | The Outcomes Were Measured in a Valid and Reliable Manner | An Appropriate Statistical Analysis Was Used | |
| Luo et al. (23) | Y | Y | Y | U | U | Y | Y | Y |
| Arsalan et al. (24) | Y | Y | U | Y | Y | Y | Y | U |
| Kurzepa et al. (25) | Y | Y | U | U | U | Y | Y | Y |
| Xu et al. (26) | Y | Y | N | Y | U | Y | Y | N |
| Perlstein et al. (27) | Y | Y | U | U | U | Y | Y | N |
| Sagheb Asl E. et al. (22) | U | Y | U | U | U | U | Y | U |
| Author | Case Control Studies | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| The Groups Were Comparable Other Than the Presence of Disease in Cases or the Absence of Disease in Controls | The Cases and Controls Were Matched Appropriately | The Same Criteria Were Used for the Identification of Cases and Controls | The Exposure Was Measured in a Standard, Valid and Reliable Manner | The Exposure Was Measured in the Same Way for Cases and Controls | The Confounding Factors Were Identified | The Strategies to Deal with Confounding Factors Were Stated | The Outcomes Were Assessed in a Standard, Valid, and Reliable Way for Cases and Controls | The Exposure Period of Interest Was Long Enough to be Meaningful | An Appropriate Statistical Analysis Was Used | |
| Herishannu et al. (28) | Y | N | U | U | Y | Y | U | Y | Y | Y |
| Gonullu et al. (29) | Y | N | U | U | Y | Y | Y | Y | Y | Y |
| Luo et al. (30) | Y | N | U | U | Y | Y | U | Y | Y | Y |
| Cojocaru et al. (31) | Y | U | U | U | Y | Y | U | Y | Y | Y |
| Muscari et al. (32) | Y | U | U | U | Y | Y | U | Y | Y | Y |
| Lagowska-Lenard et al. (33) | Y | U | U | U | Y | Y | Y | Y | Y | Y |
4.2. Description of the Included Studies
Studies that had been published between 1971 and 2018 in countries of Iran, Israel, Turkey, Pakistan, Romania, Poland, China, Italy, India, and the USA. All included studies were conducted on humans. Six studies had a case-control design (23-28), six had a cross-sectional design (22, 29-33) and one was a case series (34). Studies had been conducted between seven days to five years. The total sample size in this systematic review was 17,557 subjects for case groups and 347 for control groups. The participants, on average aged between 15 and 92 years. Bilirubin was the biomarker in all included studies.
4.3. Findings of Bilirubin Level
The results of five included studies showed an increase in the level of serum bilirubin after acute ischemic stroke, which was related to the severity of stroke (22, 25, 29, 31, 33). Three studies had reported that an increase in the level of serum bilirubin led to a reduction in stroke prevalence and improved the outcomes (24, 26, 32). Three other studies showed no significant relationship between serum bilirubin levels and ischemic stroke (28, 30, 34). Studying the influence of vitamin C on the markers of oxidative stress had shown that although vitamin C increased the levels of antioxidants, it did not improve the clinical and the functional aspects of AIS patients after three months (27). The results of the last study suggested a direct influence of the brain lesion on the polarity of the hepatic cell as a possible mechanism for the hyperbilirubinemia in patients with AIS (23). The mean total and direct bilirubin levels in patients within 24 h are indicated in Table 3. The results were significant in all studies, except for one study.
| Author | Country | Study Design | Biomarker | Age, y | Sample Size | Total Bilirubin Level in Cases | Total Bilirubin Level in Controls | Unit | Neurological Outcomes Associated with Stroke Severity (NIHSS) | Correlation Between Stroke Severity and Bilirubin | Adjusted Odds Ratio (Tbill-NIHHS) | Non-Adjusted Odds Ratio (Tbill-NIHHS) | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Case | Control | ||||||||||||||
| Total | M | F | |||||||||||||
| Herishannu et al. (28) | Israel | Case-control | Bilirubin | 46 - 80 | 73 | 55 | 18 | 63 | < 1.1 | mg/dL | + | ||||
| Gonullu et al. (29) | Turkey | Case-control | Bilirubin | 25 | 9 | 16 | 25 | 9.68 ± 5.02 | 22.05 ± 5.80 | µmol/L | + | ||||
| Luo et al. (30) | China | Case-control | Bilirubin | 15 - 92 | 608 | 383 | 225 | 188 | 18.329 | 16.147 | µmol/L | + | 1.051 | 1.084 | |
| Cojocaru et al. (31) | Romania | Case-control | Bilirubin | 73.4 ± 6.5 | 57 | 24 | 33 | 51 | 1.31 ± 0.07 | 1.04 ± 0.06 | mg/dL | + | |||
| Muscari et al. (32) | Italy | Case-control | Bilirubin | 73.2 ± 13.2 | 180 | 85 | 95 | - | mg/dL | < 10 | - | ||||
| Lagowska-Lenard et al. (33) | Poland | Case-control | Bilirubin | 72.8 ± 10.4 | 30 | - | - | 20 | 0.65 | mg/dL | + | ||||
| Luo et al. (23) | China | Cross-sectional | Bilirubin | 67 ± 0.56 | 531 | 337 | 197 | - | 18.54 ± 0.4 | Umol/l | < 10 | + | 1.005 | 3.352 | |
| Arsalan et al. (24) | Pakistan | Cross-sectional | Bilirubin | 63.5 | 44 | 26 | 18 | - | < 1.1 | mg/dL | 16.26 | + | |||
| Kurzepa et al. (25) | Poland | Cross-sectional | Bilirubin | 71.9 ± 12.1 | 43 | 22 | 21 | - | 15.4 | µmol/L | + | ||||
| Xu et al. (26) | China | Cross-sectional | Bilirubin | 65.05 | 2361 | - | - | - | < 10 | + | 1.584 | 1.866 | |||
| Perlstein et al. (27) | USA | Cross-sectional | Bilirubin | 13214 | - | - | - | 12.1 | µmol/L | + | 0.76 | 0.91 | |||
| Sagheb Asl et al. (22) | Iran | Cross-sectional | Bilirubin | 260 | 146 | 114 | 0.89 ± 0.41 | 16.5 | + | ||||||
| Bhatia et al. (34) | India | CASE sires | Bilirubin | 116 | 77 | 39 | - | mg/dL | < 10 | - | |||||
aValues are expressed as mean ± SD.
5. Discussion
This systematic review was conducted to assess the predictive value of serum bilirubin in patients with ischemic stroke. The results of the majority of included studies showed an increase in the level of serum bilirubin after acute ischemic stroke, as well as an increase in the severity of stroke. Three studies showed that an increase in the level of serum bilirubin led to a reduction in stroke prevalence and improved the outcomes of stroke. Three other studies showed no significant relationship between serum bilirubin levels and ischemic stroke. One of the studies had assessed the influence of vitamin C on the markers of oxidative stress, in which vitamin C could not improve the clinical and the functional aspects of AIS patients after three months. Finally, another study had suggested a direct influence of the brain lesion on the polarity of the hepatic cell as a possible mechanism for the hyperbilirubinemia in patients with AIS.
The results of this review revealed that, based on the majority of included studies, increased levels of bilirubin leads to increased severity of ischemic stroke. Similarly, based on another study, serum bilirubin had a significant and positive correlation with stroke severity (35).
In contrast with the findings of this review, which showed that serum bilirubin was a poor prognostic factor of ischemic stroke (25), the results of a meta-analysis including 24 case-control studies conducted in China, showed that serum levels of bilirubin might be a useful biomarker for the prediction of acute stroke (36). Similarly, another study found that the risk of all types of stroke was decreased by an increase in bilirubin level, and serum bilirubin level protected against stroke risk among male patients (37). Also, a study had reported that bilirubin concentration in plasma might be an effective marker of oxidative stress in hemorrhagic stroke patients (38). On the other hand, and in line with our results, Wang et al. indicated in their research that hyperbilirubinemia might be a biomarker for poor prognosis in the early identification of large-artery atherosclerotic stroke patients (39). Cojocaru et al. (40), in their research on 168 patients with different subtypes of acute ischemic strokes, illustrated that parameters of oxidative stress could not be used to differentiate stroke subtypes. Mendelian randomization in a study conducted in Korea in 2017 showed that serum bilirubin level was not a risk determinant for stoke (41). Similarly, the results of a research conducted by Kunutsor et al. (42) showed no significant evidence of an association between total bilirubin level and stroke risk.
The results of the three included studies showed that an increased level of serum bilirubin was linked to the reduced prevalence and improved outcomes of stroke. however, Tang et al. (43) showed in their study that high serum bilirubin level was associated with high post-stroke depression as an outcome of stroke. Results of a recent study indicated a close relationship between high levels of serum uric acid level and total bilirubin level on admission with the occurrence of major post-ischemic stroke depression within three months of stroke. However, there was an inverse relationship between 3 and 6 months post-stroke, meaning that low levels of these two biomarkers on admission led to the occurrence of major post-ischemic stroke depression, whereas six months after stroke, there was no relationship between post-ischemic stroke depression and these two biomarkers (44). Also, another research had indicated that high direct bilirubin level was not associated with discharge outcomes in ischemic stroke patients (45). A cross-sectional community-based study conducted on diabetic patients in China in 2014 aimed at assessing the influence of total bilirubin level on major complications, including stable angina, unstable angina, myocardial infarction, ischemic stroke, peripheral vascular disease, and chronic kidney disease, had indicated a significant association between total bilirubin level and lower prevalence of these complications (46). Also, based on the results of a previous study by Jorgensen et al. (47), low levels of serum bilirubin were associated with increased risk of developing non-fatal stroke as a primary cardiovascular adverse event in patients at risk for cardiovascular diseases. Similarly, Oda and Kawai (48), in their study on the Japanese health screening population, revealed that the prevalence of coronary heart disease and stroke in men, and only stroke in women, were increased as the total bilirubin level decreased. Similar results had been reported in another study (49).
5.1. Conclusions
This review showed that there was an association between the increased serum level of bilirubin and increased severity of ischemic stroke in the majority of included studies. More robust reviews and meta-analyses should be conducted to obtain a conclusion answering the controversy about the prognostic effect of the bilirubin level.