1. Background
Antimicrobial stewardship programs (ASPs) have been developed in recent years to improve the correct indication of prescribed antimicrobials and avoid resistance in all healthcare settings, including hospitals, as well as outpatients and patients from long-term care institutions (1-3). These coordinated actions could be different according to healthcare units, but usually, the core components include audit and feedback of antimicrobials, pre-approval of the target or selected antimicrobials, de-escalation according to culture results, administrative support, multidisciplinary teams, and education of healthcare staff (4, 5). Considering that infections of multi-resistant bacteria are also present in neonatal intensive care units (NICUs) and pediatric intensive care units (PICUs), the control of bacterial resistance is a global priority for the World Health Organization (WHO). Limited options of new drugs are available for children, so ASPs are interesting tools to promote the judicious use of antimicrobials (6, 7).
Interventions of ASPs have led to successful results in overall antimicrobial consumption reduction, as well as target/broad-spectrum antibiotic reduction, even when neonatal and pediatric critical care units were analyzed (8, 9). Analysis of antimicrobial use in healthcare institutions is necessary within the context of ASPs to understand the consumption patterns and establish reduction priorities by defining target antimicrobials according to local/regional profile (10). Days of therapy (DOT) or DOT per 1000 patient-days are common quantitative measures described to evaluate the amount of antimicrobial use in the pediatric population (11).
One important challenge for ASP is to evaluate the quality of antimicrobial prescriptions. From this point of view, appropriate indication means a correct prescription for a correct infectious disease syndrome, including accurate dosage and duration and avoiding broad-spectrum antimicrobials whenever possible, without damage to patient treatment (12). Most studies are focused on the detection of inappropriate use of antibiotics, with few successful interventions available to improve the quality of prescriptions in NICUs and PICUs (13, 14).
2. Objectives
This study aimed to analyze the quality of target antimicrobial requisitions after an antimicrobial policy restriction program for children.
3. Methods
3.1. Study Design and Setting
We performed an observational study in a NICU and three PICUs in Rio de Janeiro city, Brazil. The services are located inside a 135-bed pediatric hospital (Prontobaby-Hospital da Criança).
The NICU is an 11-bed unit that receives neonates from other hospitals and its own emergency service. The hospital studied does not have a delivery room or obstetric service. The PICU is a 34-bed unit subdivided into three other units according to patient profiling: PICU 1 with 10-bed capacity that receives acute critically ill patients; PICU 2 with 15-bed capacity that receives acute critically ill children, but for a short stay; and PICU 3 with 9-bed capacity that receives critically ill patients for long stay (usually with chronic diseases) or infectious diseases that necessitate isolation. All sub-units receive clinical and surgical patients referred from other hospitals or emergency services.
3.2. Antimicrobial Stewardship Program
Throughout 2016, several components of an ASP were implemented in the whole hospital, including the ICUs: Assessing the point-prevalence of antimicrobial use every three months, updating antimicrobial guidelines available, using new technologies in microbiological laboratories to accelerate the results of cultures, and more training of healthcare workers regarding the better use of antimicrobials. All ASP components were extensively discussed with healthcare teams, including administrative staff, before their full implementation.
Since 2006, the hospital’s infection control committee has actively performed healthcare-associated infection (HAI) surveillance and discussed cases related to antimicrobial use indication when requested. However, until 2016, there were neither formal hospital policies to analyze the amount and quality of antimicrobial prescribed nor training applied to clinicians on this subject.
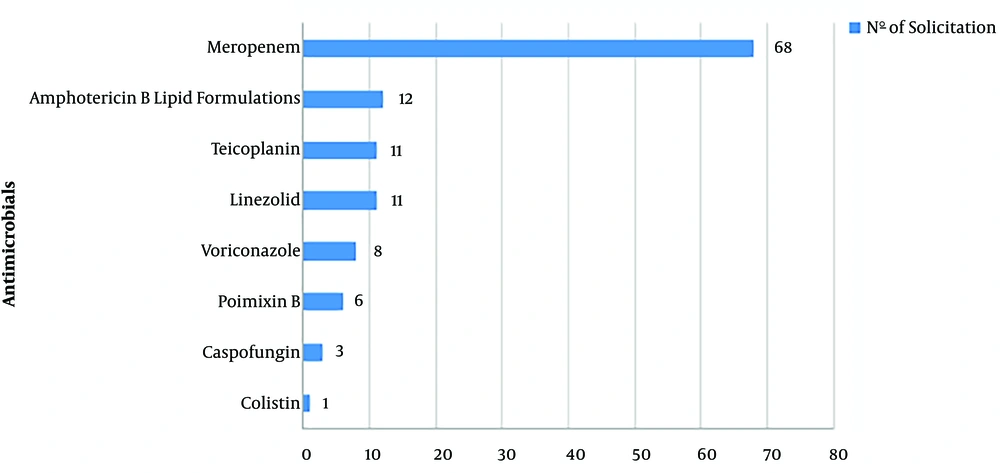
In October 2016, an antimicrobial policy restriction was formalized for 14 drugs selected by the infection control committee according to the bacterial resistance profile of the hospital and pricing, to be prescribed only after the pre-approval of a pediatric infectious disease specialist. The antimicrobials selected were amphotericin B lipid formulations, caspofungin, ceftobiprole, colistin (inhaled), daptomycin, ertapenem, imipenem, linezolid, meropenem, micafungin, polymyxin B, teicoplanin, tigecycline, and voriconazole. The flow of target antimicrobial requisition is shown in the supplementary material. In the same month, point-prevalence analysis of antimicrobial consumption was replaced by days of therapy (DOT) measurement to analyze the amounts of all antimicrobials consumed in the critical care units.
3.3. Quality Analysis of Target Antimicrobial Requisition
All requisitions regarding target antimicrobials between October 2016 and December 2017 were reviewed. We analyzed the following variables: Indication, type of antimicrobial, dose, duration, collection of cultures before administration, and the agreement of a pediatric infectious disease specialist. There were no exclusion criteria for the analysis of requisitions.
3.4. Data Analysis
We did a descriptive analysis of variables using Excel spreadsheet® version 2016 (Microsoft Corp., Redmond, WA, USA). We rated the agreement of a pediatric infectious disease specialist with target antimicrobial requisitions as excellent (90 - 100% of agreement), good (75 - 89% of agreement), moderate (50 - 74% of agreement), and poor (< 50% of agreement).
3.5. Ethical Aspects
The study was approved by the local Ethics Committee (Register 2.386.987 from 19 November 2017).
4. Results
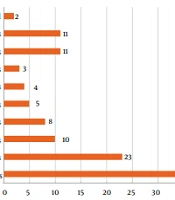
Between October 2016 and December 2017, 1,173 patients were admitted to the units, including 324 in the NICU and 849 in the PICUs. During this period, there were 164 requisitions for target-antimicrobials from the whole hospital, including 120 (73.1%) from the NICU and PICUs. Of the 14 target-antimicrobials, six of them were not requested: ceftobiprole, daptomycin, ertapenem, imipenem, micafungin, and tigecycline. Meropenem represented 56.7%, and amphotericin B lipid formulations as 10% of all requisitions. Figure 1 presents the frequency of each target antimicrobial requested.
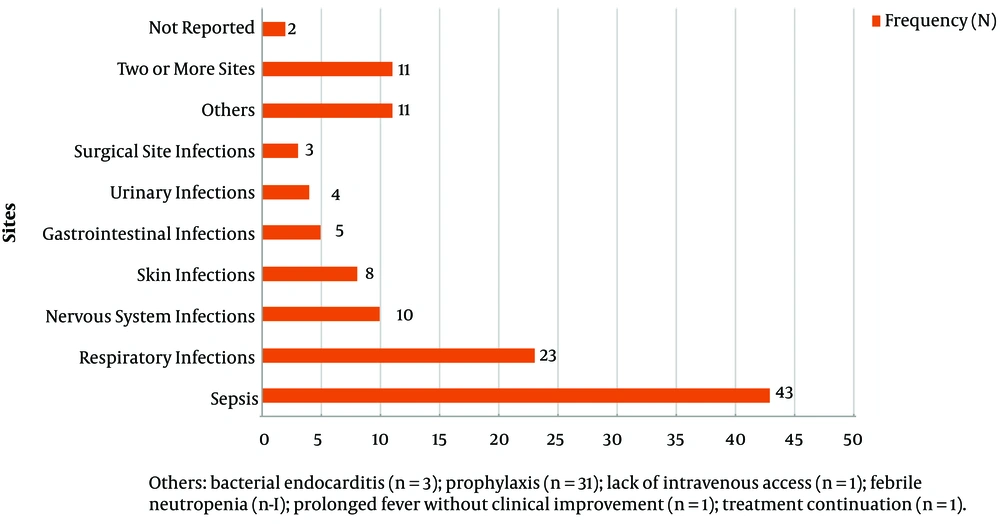
Sepsis was the most common indication for target antimicrobials corresponding to 34.1% of all solicitations, followed by respiratory infections at 19.2%, and infections in two or more sites at 9.2%. The frequency of all indications is presented in Figure 2.
In 98 of 120 (81.6%) requisitions, cultures were collected before antimicrobial administration. In 54 requisitions with cultures, the most common sites of collection were tracheal aspirate (14/98; 14.2%), blood (14/98; 14.2%), urine (11/98; 11.2%), cerebrospinal fluid (5/98; 5.1%), catheter tip (5/98; 5.1%), oropharyngeal swab (1/98; 1%), pleural fluid (1/98; 1%), surgical wound (1/98; 1%), bone marrow aspirate (1/98; 1%), and urethral secretion (1/98; 1%). In 44 cases, the site of culture collection was not reported.
On nine occasions, no cultures were collected; in eight patients, this information was not available, and in five cases, cultures were not necessary due to treatment continuation, antimicrobial prophylaxis, or lack of venous access. Considering all patients with cultures collected and patients for whom cultures were not necessary, 17 (14.2%) patients missed an opportunity to identify an infectious agent in cultures.
An infectious agent was found in 52 of 98 (53.1%) cultures collected. Gram-negative bacteria represented 50% of all positive cultures (26/52), followed by Gram-positive as 26.9% (14/52) and fungi as 23.1% (12/52). The frequency and microorganisms isolated in cultures are presented in Table 1.
| Classes | No. |
|---|---|
| Gram-negative bacteria | 26 (50%) |
| Klebsiella pneumoniae ESBL | 4 |
| Pseudomonas aeruginosa | 4 |
| Pseudomonas aeruginosa CR | 4 |
| Klebsiella pneumoniae | 3 |
| Klebsiella pneumoniae CR | 2 |
| Stenotrophomonas maltophilia | 2 |
| Acinetobacter lwoffii | 2 |
| Enterobacter sp ESBL | 2 |
| Others a | 3 |
| Gram-positive bacteria | 14 (26.9%) |
| Coagulase-negative staphylococcus | 5 |
| Staphylococcus epidermidis MR | 3 |
| Staphylococcus aureus | 2 |
| Micrococcus | 1 |
| Enterococcus faecalis | 1 |
| MRSA | 1 |
| Streptococcus mitis | 1 |
| Fungi | 12 (23.1%) |
| Candida sp | 8 |
| Candida albicans | 3 |
Abbreviations: ESBL, Extended-spectrum Beta-lactamase; CR, Carbapenem-resistant; MRSA, Methicillin-resistant Staphylococcus aureus.
aSerratia liquefaciens (1), Escherichia coli ESBL (1), Burkholderia cepaciae (1)
Besides, 111 requisitions were approved by pediatric infectious disease specialists, corresponding to 92.5% of the total. The rate was considered to be excellent. Of the nine remaining refused requisitions, on five occasions, the case was discussed further, leading to the prescription of a narrow-spectrum antimicrobial; four were refused due to lack of information and in the last one, de-escalation of the antimicrobial was possible. No mistakes regarding dose and duration were found.
5. Discussion
Inappropriate antimicrobial prescription represents poor quality of good practice to reduce consumption and help avoid bacterial resistance. Several reports around the world identified a high rate of inappropriate antimicrobial prescription (15, 16). The main problems identified are an unnecessary indication, wrong antibiotics chosen, wrong directions, incorrect posology, prolonged prescriptions, and the use of agents with an excessively broad coverage spectrum (17-19).
In our article, we proposed to analyze the quality of target antimicrobial requisitions after the full implementation of an ASP in the NICU and PICUs where empiric and broad-spectrum antimicrobials are usually necessary to use, due to the high risk of death as a consequence of serious infections. The reduction of antimicrobial consumption with a high rate of quality in prescriptions is possible and reported in the NICUs (20).
To achieve the best possible impact of our ASP, an extensive preparatory phase was conducted before its full implementation, where all healthcare workers involved in the process presented their workflow and contributed to the program with valuable suggestions to work with little interference with daily practice. The engagement of clinicians is considered to be vital for actions related to the improvement of antibiotic use in hospitals (12).
Sepsis and respiratory infections were the most common reasons for the requisition of target antimicrobials. This finding agrees with previous studies that described indications for antimicrobial use in neonates and children (21). In a point-prevalence study of antimicrobial use conducted in 226 hospitals from 41 countries, the most common reason for treating children was bacterial lower respiratory tract infection (18.7%), and sepsis was the main reason for treating neonates (36.4%) (21). Sepsis was also the most common reason for an indication for antimicrobial therapy in a long-term series in Sweden (22).
Gram-negative bacteria, especially Extended-spectrum Beta-lactamase (ESBL) producers, represented important agents of healthcare-associated infections in the hospital studied, and for this reason, empiric treatments, including meropenem, were necessary. Carbapenems are a broad-spectrum antibiotic class used for critically ill patients admitted to intensive care units with infections due to ESBL producers, but their indiscriminate use could increase resistance, leading to untreatable infections due to lack of treatment options (23). To preserve all carbapenem activities in our institution, this class was included to be used in the whole hospital, only after discussion and the agreement of pediatric infectious disease specialists. Restricted antimicrobial lists frequently include carbapenems as a class to be used in selected infections due to multidrug-resistant bacteria, included as part of a national plan for antibiotic restriction (24-26).
Our study found a high positive culture rate (53.1%). Although several studies present a blood culture positivity rate around 10%, (27) it is possible to highlight two factors that probably contributed to this result: Cultures were collected from diverse sites, not only blood; and beyond that, most were collected from critical patients for whom were needed broad-spectrum antimicrobials.
Despite the high rate of culture collections in patients that required target antimicrobials, at least 14% of them missed the possibility of an infectious agent identification. These “lost opportunities” are avoidable, for example, with the de-escalation of broad-spectrum antimicrobials in favor of narrow-spectrum drugs with the same effectiveness but with less possibility of resistance induction.
In terms of pediatric infectious disease specialist agreement with target antimicrobial requisitions, the rate was considered excellent, reaching more than 92%, which demonstrates the high capacity of clinicians for identifying correctly the indications for a broad-spectrum antibiotic, including important aspects involved in the quality of prescriptions, such as dose and duration. Our rate was similar to that reported by Luthander and Cols, who found 98.5% (273/277) of appropriateness in antimicrobial use during a 2003 - 2010 survey in a Swedish pediatric hospital, despite that this report analyzed all hospital wards, not only ICUs.22 Quality programs also contribute to the improvement rate of compliance with guideline recommendation/correction antibiotic administration for selected infectious disease syndromes, such as community-acquired pneumonia or critically ill children assisted in emergency departments (28, 29).
This report has some limitations. First, it was conducted in a single-center where it was possible to control factors that interfered with the higher compliance of clinicians to correct indication of target antimicrobials. We believe that more studies using a similar approach involving multiple healthcare institutions could confirm our positive results. Another limitation was the short observation period in which the compliance of clinicians was considered excellent. Further reports should confirm the sustained high quality of target/broad-spectrum antimicrobial prescriptions and ASPs influence on mortality related to infectious diseases over a longer observation period. Finally, previous data about the appropriateness of antimicrobial prescription before intervention were not available to compare the effects in two periods (before and after).
In conclusion, we found a high-quality rate of target antimicrobial requisition. Antimicrobial policy restriction could contribute to improving the quality of antimicrobial prescriptions, even in the NICU and PICUs.