1. Background
Candidemia in neonates and children can lead to severe morbidity, and it can be even fatal despite appropriate treatment (1). According to the literature, the epidemiologic pattern and frequency of pediatric candidal infection differs from adults (2); thereby needing comprehensive strategies to the management of candidemia (2). Candida spp. is known as the third to fourth most common pathogen cause of nosocomial bloodstream infections (BSI) (3, 4). This destructive infection is mainly caused by, Candida albicans (5, 6). Currently, the epidemiology of the candidemia is changing, and we are facing the emergence of non-C. albicans (NAC) spp., including Candida parapsilosis, Candida tropicalis, Candida glabrata, and Candida krusei (7). Candida parapsilosis is considered the predominant causative agent of candidemia in pediatric oncologic disorders (2). Differences in Candida spp. are related to disease severity and diversity in antifungal susceptibility, patterns; therefore, understanding the type of species is clinically important.
Only limited information about burden of infection and risk factors are available in neonates and pediatrics in Iran and further study is required to address its burden (5, 6, 8-10).
2. Objectives
The present study was conducted to raise attention to the frequency of Candida spp. and evaluation of risk factors in hospitalized neonates and children that were suspected of having invasive candidiasis (IC).
3. Methods
3.1. Study Areas and Subjects
This cross-sectional study was conducted from 2017 to 2018. The blood samples were obtained from each patient using a sterile syringe and directly infused into biphasic blood media. Seventy-five blood culture samples were collected from neonates and children ≤ 14 years old who were hospitalized and suspected of fungal infection. Bacterial positive samples were excluded from the study.
3.2. Conventional Identification
Blood samples were inoculated into aerobic culture medium bottles (BACTEC Peds Plus/F Culture Vials, Ireland) followed by incubation for a period of 5 days in the automated blood culture system BACTEC 9120 (Becton Dickinson, Spark, MD, USA). The candidemia was defined as having more than one positive culture specimen. Each episode of IC was considered a new case if at least one month would have been passed from a previously treated occurrence (3). The positive cultures were subcultured on blood agar (Merck, Darmstadt, Germany) at 35°C and checked each day for up to five days. To access the pure colony, the positive cultures were subcultured on CHROMagar (CHROMagar Paris, France) at 35°C for 24 hours.
3.3. Molecular Identification
All isolates were cultured on yeast extract peptone dextrose (YEPD) agar (Merck, Germany) at 37°C for 24 hours. DNA was extracted using the genomic DNA extract kit (GeneAll, Korea).
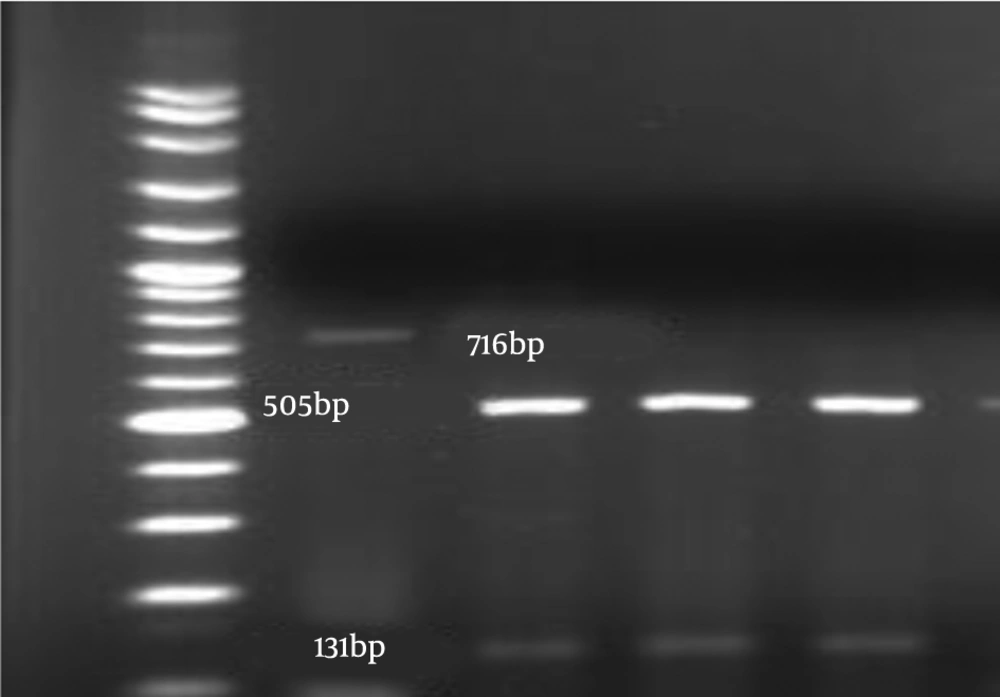
Definite identification of Candida spp. was confirmed by the PCR-restriction fragment length polymorphism (RFLP) approach based on the amplification of ITS1-5.8 SrDNA-ITS2 region with pun fungal primers ITS1-ITS4 that were synthesized by Bioneer Company (Korea) and enzymatic digestion. The PCR was conducted with a mixture included 1 µL of extracted DNA, 10 µL of Taq DNA Polymerase Master Mix RED (Ampliqon, Denmark), 1 µL of each ITS1 (5’-TCCGTAGGTGAACCTGCGG-3’) and ITS4 (5’-TCCTCCGCTTATTGATATGC -3’) pair primers (11), H2O up to 25 µL. The PCR program as follow as; 95°C for 5 minutes, 35 cycles of denaturation for 5 minutes at 94°C, annealing for 1 minute at 58°C, an extension for 1 minute at 72°C, with a final extension of 7 minutes at 72°C. The amplicons were visualized using 1.5% agarose gel electrophoresis in TBE buffer (Merck, Germany) and stained with safe stain (Waltham, USA). The products were subjected to the MSP1 restriction enzyme (Fermentas, USA). Restriction fragments were separated by 2% agarose gel electrophoresis for 1 hour. The C. albicans complex and C. parapsilosis complex were differentiated using the HWP1 gene amplification and PCR-RFLP with NlaIII restriction enzyme, respectively (12, 13). The PCR program was similar to ITS1-5.8 SrDNA- ITS2 PCR with a minor difference in annealing temperature.
4. Results
Out of 75 blood cultures, 42 (84%) cases were positive for Candida spp. growth of whom 30 (71.42%) were female, and 12 (28.57%) were male. Thirty-two (76%) candidemia were presented in pediatrics with 6 years up to 12 years, 10 (23.80%) in neonates of one month or less. The patients had received several wide-spectrum antibiotics. There was evidence of empiric antifungal treatment based on medical records, including caspofungin, voriconazole, amphotericin B and it was not qualified for antifungal therapy. Nineteen (45.23%) of pediatrics had hematological malignancy and 2 (7.1%) had heart disease and remains had other non-leukemia related disease or neonate. The evidence of neutropenia, low weight < 1500, long-stay hospitalization, chemotherapy, history of surgery, central venous catheters (CVC) use were also seen.
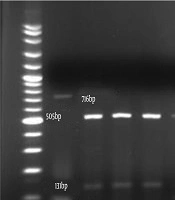
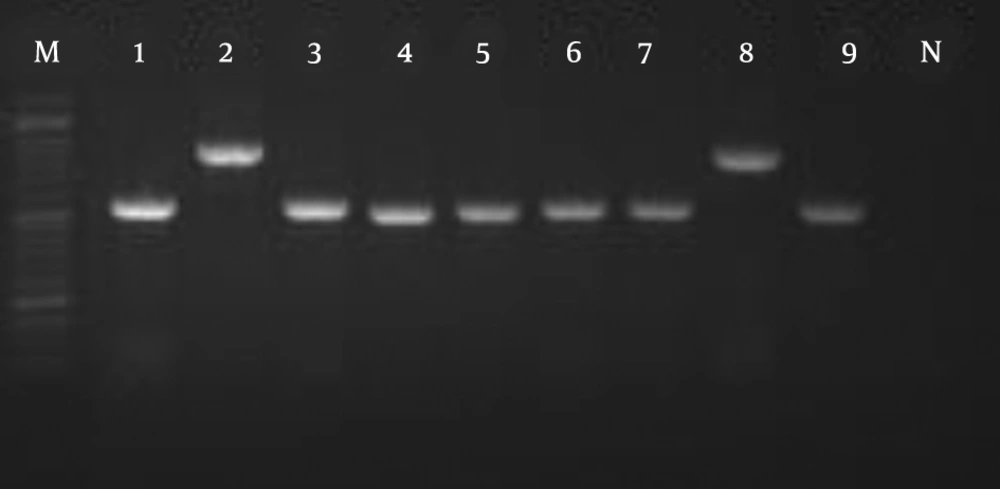
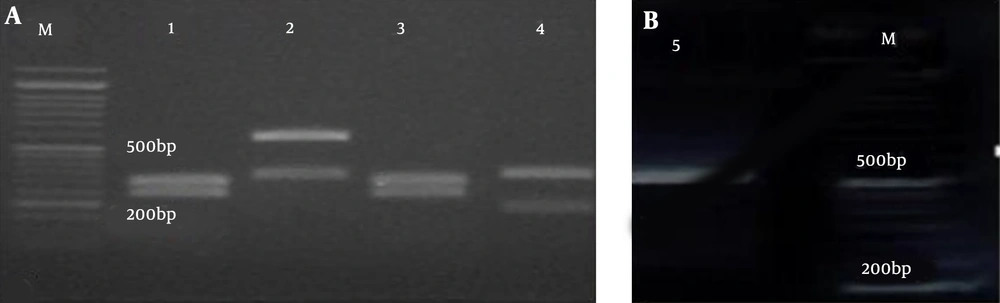
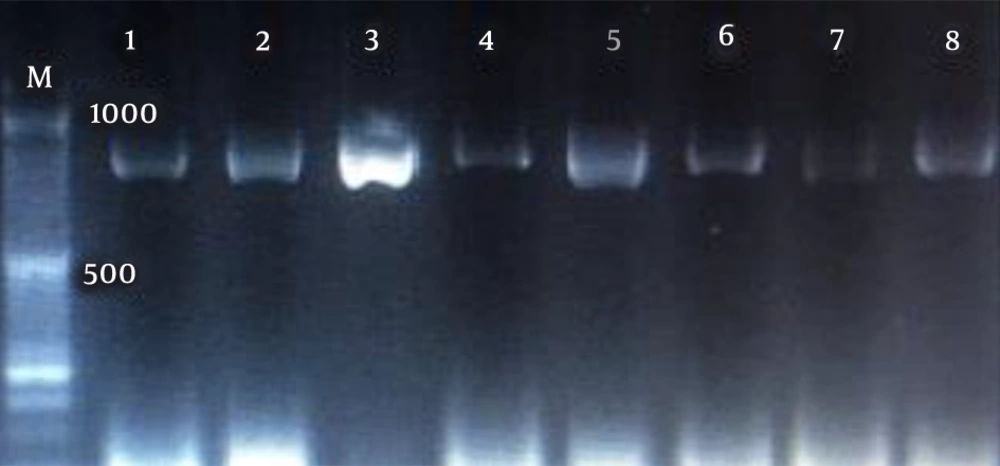
The amplification pattern of the ITS1-5.8 SrDNA-ITS2 region of Candida spp. using the ITS1-ITS4 primers is shown in Figure 1. Subsequently, all isolates were subjected to the digestion by MSPІ enzyme to precise identification (Figure 2). All of the C. albicans complex strains were identified as C. albicans with HWP1 gene primers (Figure 3).
In the present study, C. parapsilosis (n = 25; 59.52%) was known as the most prevalent isolated species followed by C. albicans (n = 11; 26.19%), C. tropicalis (n = 4; 9.52%), C. glabrata (n = 2; 4.76%) (Figure 4).
5. Discussion
The number of uncommon Candida spp. isolated from clinical samples is increasing, and invasive candidiasis (IC) caused by these species may paradoxically be the result of advances in medical care (6). Regarding higher resistance to fluconazole and echinocandins in non-albicans Candida spp. (NAC), identification of Candida spp. is necessary for appropriate management of infection.
As mentioned before, the studied population had several risk factors for developing candidemia. In the present study, C. parapsilosis (n = 25; 59.52%) known as the most prevalent isolated species followed by C. albicans (n = 11; 26.19%), C. tropicalis (n = 4; 9.52%), and C. glabrata (n = 2; 4.76%). These findings were consistent with previous reports that indicated C. parapsilosis complex was considered the predominant causative agent of candidemia in both neonates and pediatrics (5, 9, 10, 12, 13). In some multicenter studies, C. albicans was considered the most frequent species in pediatrics (6, 8, 10).
Regarding the risk factors, there was no significant difference between the two groups of candidiasis by C. albicans and NAC (P < 0.01). A study by Juster-Reicher et al. also revealed no difference between the two groups with candidemia caused by C. albicans and C. parapsilosis (P < 0.01) (14) considering the risk factors such as CVC, oncologic disorders, and chemotherapy. However, there are several studies showing a higher frequency of bloodstream infections caused by C. parapsilosis in CVC usage (5, 12, 15-17). In contrast to the pediatric population, low birth weight (LBW), and very low birth weight (VLBW) neonates were more associated with candidemia caused by C. parapsilosis (16, 18).
In contrast to previous studies (6, 19) in pediatric C. tropicalis is the third common strains were isolated in candidemia in the present study. In line with previous research, further analyses revealed that C. tropicalis candidemia has been associated with neutropenia and leukemia (20).
C. glabrata is rarely seen in neonates and children. However, in a study performed on immunocompromised children, C. glabrata was isolated as the second frequent cause of candidemia (19). Contrary to Charsizadeh et al. study (6), we did not isolate C. glabrata in blood samples in the neonatal intensive care unit (NICU). Two C. glabrata strains were isolated in patients suffering from acute lymphoblastic leukemia (ALL), the later admitted in pediatric intensive care unit (PICU) and both of them had CVC. Kara et al. (18) isolated C. glabrata from pediatrics that had infective endocarditis. In a study from Australia, neonates had a higher rate (9.1%) than pediatrics (10). Since Candida spp., which were once considered to be harmless, can now cause candidemia and significant morbidity and mortality in patients residing in intensive care units, identifying the underlying conditions and risk factors must be noticed seriously. Candida bloodstream infection might be the result of selective pressure of some antifungal agents, previous wide spectrum antibacterial therapy, multiple intravascular catheters, parenteral nutrition, and mechanical ventilation. However, some studies found no relationship between Candida serotypes and underlying diagnosis, risk factors, clinical features, and outcomes (20-23).
5.1. Conclusions
According to potentially dangerous complications of bloodstream infection by Candida spp. in neonates and children, it is necessary to identify and eliminate the underlying conditions and risk factors of this disease within an appropriate framework.