1. Background
Sickle cell disease (SCD) is a chronic hemolytic hereditary anemia, resulting from a mutation in which valine substitutes for glutamic acid in codon 6 of the β-hemoglobin chain, thereby leading to the formation of hemoglobin S. This basic defect is the main cause of the various clinical signs and symptoms of this disease (1-3). The combination of hemoglobin S (HbS) with another abnormal hemoglobin, such as hemoglobin C (HbC), beta-thalassemia, and HbS leads to various hemoglobinopathies such as HbSC, HbS beta-thalassemia, and HbSS, which is known as sickle cell anemia and it is the most severe form of sickle cell disease (1-3). Nigeria is the country with the highest-burden of the disease, and it remains a public health problem because of its high prevalence (4). Sickle cell disease has high morbidity and mortality and it is characterized by acute clinical symptoms. Notable problems among these events are severe anemic episodes requiring blood transfusion and painful vaso-occlusive episodes among others.
Viral hepatitis is a major health problem in developing countries. Hepatitis B virus (HBV) and hepatitis C virus (HCV) can cause high morbidity and mortality among chronic infections. HBV is a DNA virus that is responsible for HBV infection, which can cause severe and life-threatening liver disease (5). It is common in Asia, Africa, China, and the Middle East (6, 7). It is one of the most common infectious diseases globally (8). World health organization (WHO) estimates that HBV results in two million deaths each year worldwide and 230,000 of these occur in Africa (9). The HCV is a single-stranded RNA virus with a propensity for causing chronic and debilitating illness that may result in significant morbidity and mortality. It affects about four million people in the USA, and it is the most common cause of chronic liver diseases in the country leading to about 10,000 deaths per year (5).
HBV and HCV are present in the blood, saliva, semen, vaginal secretions, menstrual blood, and to a lesser extent, perspiration, breast milk, tears, and urine of infected individuals (5, 10). The menace of these infections is being decreased in the developed countries by proper screening and timely application of appropriate treatment. However, despite improved screening measures of blood products and fairly widespread immunization in Nigeria, these infections still pose a huge threat to at-risk individuals because of illegal/inappropriate use of blood products and the promotion of some unsafe cultural/traditional (unorthodox) practices in the country (4, 7, 11). The chronic nature of SCD may make the caregivers of children with SCD seek help for their wards from givers of unsafe medical and unorthodox practices, thereby exposing the patients with SCD to hepatitis B and C infections. In addition, the frequent use of blood products for patients with SCD further put them at risk of contracting these infections (4). Studies on hepatitis infections among pediatric patients with SCD are scanty in Nigeria and there is no information about the study location. This study was carried out to determine the prevalence of HBV and HCV infections among children with SCD in comparison to their age and sex-matched controls. In addition, the associated sociodemographic features and risk factors for HBV and HCV infections were examined.
2. Objectives
The findings of this study could help policymakers, healthcare providers and other stakeholders in understanding the dynamics of hepatitis infections in our study locality and suggest appropriate solutions.
3. Methods
This study was a cross-sectional study conducted at the pediatric sickle cell disease and children follow-up clinics of the Ekiti State University Teaching Hospital, Ado-Ekiti, southwest Nigeria. The hospital is a state government-owned hospital that was established in April 2008 to serve as a tertiary and referral health center for the secondary and primary tiers of health facilities in Ekiti State. All consecutive patients with SCD who were in a steady-state and who agreed with the study or whose parents gave informed consent to participate in the study were recruited over 6 months (from March 2016 to August 2016). The controls were children with non-SCD who attended the children out-patient clinics of the hospital for routine medical care who agreed with the study or whose parents gave informed consent to participate in the study. Using the formula for calculating sample size for prevalence studies (12, 13). Sixty participants were required for this study. We, however, enrolled 116 patients with SCD and 116 age- and sex-matched controls. A proforma was used to record the information obtained from each participant. The information included their sociodemographic characteristics, occupation of the parents, parents’ highest level of formal education, detailed history of previous interventions (both hospital and non-hospital based), history of hepatitis B vaccination, awareness of HBV infection, and hemoglobin genotype. The Hb genotypes of the participants were confirmed by Hb electrophoresis and high-performance liquid chromatography (HPLC), Biorad, USA, Variant II, using the Beta thalassemia short program and screening for hepatitis B and C antigens by Enzyme-linked Immune-sorbent Assay (ELISA) using 1ml of blood obtained by needle prick. The hepatitis surface antigen kits manufactured by Biotech Laboratories, USA, were used and the tests were carried out according to the manufacturer’s instructions. This kit has been validated by previous studies in Nigeria (14). Social classes of the parents were determined using the Oyedeji classification system (15), which used the formal educational attainment and occupation of parents.
3.1. Ethical Consideration
Ethical approval was obtained from the Ethics and Research Committee of the Ekiti State University Teaching Hospital, Ado-Ekiti, Ekiti State, Nigeria.
3.2. Data Analysis
Data were analyzed using the Statistical Package for Social Sciences (SPSS) version 20.0. Descriptive statistics were used and frequencies of the various sociodemographic factors presented as tables. Proportions were used to determine the prevalence of HBV and HCV infections. A P value of < 0.05 was regarded as statistically significant.
4. Results
The study involved 116 patients with SCD (107 hemoglobin SS and 9 hemoglobin SC) and 116 patients with non-SCD (96 hemoglobin AA and 26 hemoglobin AS) age- and sex-matched controls.
4.1. Sociodemographic Characteristics of the Study Participants
The mean ages (Standard deviation (SD)) of the patients with SCD and controls were 8.35 (4.50) years and 8.92 (3.25) years, respectively. There was no statistically significant difference in the gender of patients and controls (P = 0.89); however, most of the patients with SCD belonged to the low socioeconomic class (Table 1).
| Characteristics | SCD Patient (N = 116), No. (%) | Control (N = 116), No. (%) | P Valuea |
|---|---|---|---|
| Sex | 0.89 | ||
| Male | 75 (64.7) | 74 (63.8) | |
| Female | 41 (35.3) | 42 (36.2) | |
| Social class | 0.04 | ||
| Low | 67 (57.8) | 57 (49.1) | |
| Middle | 45 (38.8) | 45 (38.8) | |
| Upper | 4 (3.4) | 14 (12.1) |
Sociodemographic Characteristics of the Study Participants
4.2. Knowledge of Hepatitis B and C Infection Among the Patients with SCD and Controls
More (94.8%) caregivers of patients with SCD were aware of HBV compared with caregivers of the controls (71.6%). This difference was statistically significant (P < 0.001) (Table 2). There was no significant difference between the two groups (P = 0.15) in terms of the parents’/caregivers’ knowledge about HBV and HCV status of their children (Table 2). Similarly, there was no significant difference between the two groups in terms of HBV vaccination (P = 1.00) (Table 2).
| Parameter | SCD Patients (N = 116), No. (%) | Control (N = 116), No. (%) | P Value |
|---|---|---|---|
| Aware of hepatitis B and C infections | < 0.001a | ||
| Yes | 110 (94.8) | 83 (28.4) | |
| No | 6 (5.2) | 33 (71.6) | |
| Are you aware of hepatitis B and C status of your child | 0.15a | ||
| Yes | 20 (17.2) | 30 (25.9) | |
| No | 96 (82.8) | 86 (74.1) | |
| Hepatitis B vaccination | 1.00b | ||
| Yes | 114 (98.3) | 114 (98.3) | |
| No | 2 (1.7) | 2 (1.7) |
Participants’ Vaccination Status and Caregiver Awareness of Hepatitis Status of Their Wards
4.3. Risk Factors for HBV and HCV Infections Among Study Participants
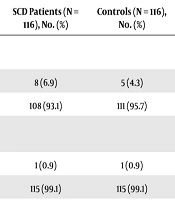
Most children with SCD received blood transfusion compared with controls. This difference was statistically significant (P = 0.001) (Table 3). However, there was a difference between the two groups in terms of the rate of scarification and surgical operations (Table 3).
Risk Factors for HBV and HCV Infections Among Study Participants
4.4. The Prevalence of HBV and HCV Infection
The seroprevalence of HBV among the patients with SCD and controls was 1%. One patient in each group (patients with SCD and controls) had a hepatitis B surface antigen (HBsAg). Hence, there was no difference in the seroprevalence rate of HBV between the two groups. None of the study participants was positive for HCV (0% amongst the two groups). The two patients who were seropositive for HBV received blood transfusions and scarification marks. The first patient with SCD was initially transfused at a private facility before being diagnosed with HBV infection when she was being transfused at our facility following an anemic crisis. The second patient (control) was 3 years old with post meningitis hydrocephalus who received blood transfusion only in a private facility and several scarifications at unorthodox centers. The two infected children could not do further investigations like HBeAg and viral load that would determine the appropriate intervention. Table 4 shows the characteristics of HBV seropositive children.
| Characteristics | SCD Patient | Controls |
|---|---|---|
| Age | 15 years | 3 years |
| Sex | Female | Female |
| Caregivers were aware of hepatitis virus | Yes | No |
| Caregivers knew hepatitis B and C status of child | Yes | No |
| Social socioeconomic class | Low | Low |
| Received hepatitis B vaccine | No | No |
| Had received scarification marks | Yes | Yes |
| Place where scarification marks were administered | Traditional healing home | Traditional healing home |
| Had received blood transfusions | Yes | Yes |
| Number of blood transfusions received | Two | One |
| Place where blood transfusions was/were received | Private facility/EKSUTH | Private facility |
Characteristics of the Hepatitis B Seropositive Patients
5. Discussion
In this study, we found a low seroprevalence of HBV infection (1%) among each group of the patients with SCD and non-SCD controls. Also, none of the children had HCV infections (0%). This low seroprevalence of 1% is comparable with the findings of George and Yaguo Ide in Port Harcourt, Nigeria in 2007 who reported that only 3 patients (3.6%) out of 84 patients with SCD were positive-HBsAg and 1 (1.0%) patient among their 100 controls (aged and gender-matched hemoglobin AA children) was positive-HBsAg (7). However, our prevalence of 1% among each group of the patients with SCD and non-SCD controls is much lower than the reports of Jibrin et al. in Sokoto, Nigeria in 2011 where 17.3% of patients with SCD and 10.7% of controls were HBV seropositive (16). Similarly, an earlier study by Abiodun et al. in Benin Nigeria also reported a higher prevalence of HBV infection rate of 39.2% among patients with SCD and 19.3% among the controls (17). Differences in the prevalence rates between our study and those from Sokoto and Benin may be due to the exclusion of hepatitis B vaccinated children by the Sokoto study while the Benin study was conducted prior to the introduction of hepatitis B vaccination into the National Programme on immunization (16, 17).
Hence, the low seroprevalence of HBV infection found in our study may be a reflection of the gradual decline of HBV infection among children in our environment due to the effect of the high coverage of hepatitis B vaccination. This is further explained by the fact that 98.7% of our study participants received hepatitis B vaccination, and none of the two children who were HBsAg seropositive received the HBV vaccine. This observation further points to the protective effect of vaccination against the development of the infection. Furthermore, Uleanya et al. in Enugu, Nigeria earlier observed the association between HBV vaccination and declining the prevalence of HBV infection in children (18).
The two children who were seropositive for HBV infection in this study had blood transfusions at private facilities before being diagnosed with the infection at our facility when they were to be transfused. There is the need for more education of health care providers at the peripheral centres with regards to safe blood transfusion practices. It is also important that these healthcare providers adhere to standard practices because most patients are likely to present at their facilities before reaching tertiary care centers like the current study setting. However, it must be noted that it is difficult to attribute the HBV infection of the patients to the care received at private facilities because both of them also had scarifications done at unorthodox centers, which could predispose them to HBV infection. Similarly, although the 15-year-old patient with SCD who was seropositive for HBV infection denied sexual activity, the possibility of sexually transmitted infection as the source of her being HBV seropositive cannot be ruled out. Nevertheless, these observations call for a general education of the populace on how to avoid practices that could predispose to hepatitis infections.
For the 3-year-old, it is possible that the source of the HBV infection can either be from the blood transfusion or the scarifications received from unorthodox centers because of the chronic nature of the child’s medical condition. Given that the practitioners of unorthodox medicine in Nigeria and other developing countries are a very large but yet informal group of healthcare providers, there is a need to educate them on how to avoid unsafe practices that are inimical to the health of their clients. One solution is suggested to the government of various developing countries to first recognize this group of healthcare providers and then enlist and train them for safe alternative or complementary medical care.
More SCD caregivers compared to the controls (94.8% versus 71.6%) were aware of hepatitis infections, and this may be due to the nature of SCD where patients tend to have frequent contact with the hospital facility as a result of their disease complications. Therefore, they may have increased knowledge about health-related issues, probably because of the numerous health education that they might have received from health workers during their numerous visits to the hospital. However, our finding is inconsistent with the reports of Okonkwo et al. in Calabar, Nigeria, who assessed the level of knowledge of some Nigerian adults about HBV infection and found that only 44.2% of the study participants had any knowledge about HBV (19). Their study may, however, be a reflection of the level of knowledge of HBV in the community.
Although most caregivers of children with SCD were aware of HBV, only 17.2% of them knew their wards’ HBV status. Among the control population, only 25.9% of them knew the HBV status of their children/wards. This low knowledge of HBV status among our study participants might reflect the possibility of generally poor knowledge of HBV status in our community. Our finding of low awareness of HBV status is consistent with the study of Abiodun et al. in Nigeria who found that about 80% of their participants, who were cleaners in a tertiary hospital, reported not being aware of their HBV status (20). This is not surprising giving the fact that HBV screening is not free in Nigeria at the moment. Hence, given the high poverty rate in the country (21), the government may need to make a hepatitis screening test free for the populace to allow early diagnosis and necessary interventions.
Furthermore, the inability of infected children to undergo further investigations required for appropriate intervention due to the lack of adequate tools at our facility raises the need for stakeholders to prioritize care regarding liver-related problems at our facilities. These costly investigations are only available at distant privately owned centers away from our facility. This further affirms the high financial burden of SCD on caregivers and their wards (21).
The characteristics of patients with hepatitis infection in this study are not different from what has been described in the literature as scarifications, and blood transfusions are documented risk factors for contracting hepatitis infections (4, 7, 9, 16-18). Therefore, more efforts should be made to control these risk factors. In conclusion, the seroprevalence of HBV and HCV infections among pediatric patients with SCD is very low in this study and this may be due to the impact of HBV vaccination because only unvaccinated children were infected.
5.1. Limitations
The HBV status of the mothers of our study participants is not known, hence, the possibility of our infected patients contracting the infections from their parents via mother to child (vertical) transmission cannot be ruled out. In spite of this, this study provides information about the seroprevalence of HBV and HCV infections among patients with SCD in our study area. It also highlights some dangerous practices that may increase hepatitis infections among SCD patients.