1. Background
Bacterial meningitis (BM) is one of the most severe infectious diseases that affect children, with a high mortality and morbidity rate. The most prevalent causes of community-acquired BM in children are Streptococcus pneumoniae, Neisseria meningitidis, and Haemophilus influenzae type b (Hib) (1-4). Other bacterial etiologies such as Salmonella enterica are uncommon causes of BM in children beyond the neonatal period (5, 6).
Rapid and accurate diagnosis of BM is crucial to start effective treatment and reduce its complications, sequels, and mortality (3, 4). The World Health Organization (WHO) has defined some criteria for BM diagnosis and surveillance, which are frequently applied, particularly in developing countries (7, 8). A probable case is defined as having clinical findings suggestive of BM, such as the sudden onset of fever or headache, stiff neck, or consciousness changes, as well as one of the following signs: neck stiffness, altered consciousness, or other meningeal signs, besides microscopic and chemical analysis of cerebrospinal fluid (CSF). The CSF examination should show at least one of the following: turbidity or leukocytosis (> 100 cells/mm3), leukocytosis (10 - 100 cells/mm3), and either an increased protein (> 100 mg/dL) or decreased glucose (< 40 mg/dL) (9, 10). The BM diagnosis should be confirmed by isolating a bacterial pathogen from an ordinarily sterile clinical specimen such as CSF or blood using the culture method, detecting a bacterial antigen in normally sterile fluids (i.e., CSF or blood), or Gram staining (11, 12).
The CSF culture is a gold standard laboratory method to confirm BM and a valuable test to target empirical therapy. However, it has some critical limitations (13). Culture results take at least 48 - 72 hours to be reported (14). Obtaining CSF samples after commencing antibiotics, poor techniques in collecting and handling samples to a laboratory, and poor testing techniques in the laboratory, especially in developing countries, may reduce CSF culture's sensitivity to only 30% (15). In recent years, it has been demonstrated that molecular amplification methods, particularly PCR, have a high sensitivity and specificity in BM diagnosis (16, 17).
2. Objectives
We aimed to determine the sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) of WHO criteria and multiplex real-time PCR for diagnosing childhood BM in a tertiary referral hospital in Shiraz, southern Iran. A blood or CSF culture or multiplex real-time PCR for identifying S. pneumoniae, N. meningitidis, and Hib confirmed the BM diagnosis. Patients’ CSF was also investigated for seven common causes of viral meningitis by real-time PCR.
3. Materials
Totally, 492 CSF samples were collected from children (aged one month to 17 years) who were clinically suspected of BM and admitted to the pediatric wards of Nemazi Teaching Hospital affiliated to Shiraz University of Medical Sciences in Shiraz, southern Iran, between August 2016 and September 2017. The CSF samples of these patients were sent to the Professor Alborzi Clinical Microbiology Research Center. All patients with more than five white blood cells (WBC) per microliter in CSF were included. We used a questionnaire to collect demographic, clinical, and laboratory data from patients’ medical records retrospectively. The study was approved by the Ethics Committee of Shiraz University of Medical Sciences, and informed consent was obtained from parents or legal guardians of children who participated in the study.
The clinical findings in favor of meningitis included the acute onset of fever (> 38.5°C rectal or 38.0°C axillary), seizure, photophobia, neck stiffness, and altered consciousness. In our center, the child’s father or legal guardian needs to give written consent for performing a lumbar puncture (LP). We excluded immunocompromised children, developed meningitis after head trauma or surgery on the central nervous system and ventriculoperitoneal shunts. Bacterial meningitis was assigned to patients with clinical and laboratory evidence of meningeal inflammation with positive Gram staining, routine bacterial culture, or bacterial PCR test results. Aseptic meningitis was assigned to patients with clinical and laboratory evidence of meningeal inflammation with negative Gram staining, routine bacterial culture, and bacterial PCR test results. Based on the WHO criteria, BM refers to patients with clinical findings in favor of BM and at least one of the following: (1) leukocytosis in CSF (> 100 cells/mm3), (2) leukocytosis (10 - 100 cells/mm3) and either an elevated protein (> 100 mg/dL), decreased glucose in CSF (< 40 mg/dL), or isolation of a bacterial pathogen from blood culture, and (3) identification of a bacterium in the CSF by Gram staining or isolation of a bacterial pathogen in CSF culture (10, 11).
All patients were routinely investigated and treated by attending physicians. The usual empirical antibiotics for treating BM in our center included the combination of vancomycin and ceftriaxone. In addition, dexamethasone was routinely prescribed intravenously (0.15 mg/kg every six hours for two days) in BM children (17). The microscopic examination of CSF was done, including total white blood cell (WBC) count with differential. Laboratory tests were performed according to the standard laboratory methods, including CSF/serum glucose levels, CSF protein levels, serum C-reactive protein (CRP), ESR, Gram staining, and culture. We stored 0.5 milliliters of the CSF sample at -20°C to perform PCR tests.
For DNA extraction from CSF, 200 µL of the specimen was added to 100 µL of Tris-EDTA buffer containing 0.04 g/mL lysozyme and 75 U/mL mutanolysin (Sigma Chemical Co.) and incubated for one hour at 37°C. Then, DNA extraction was performed following the proteinase K phenol-chloroform protocols. Taqman Multiplex real-time PCRs for detecting N. meningitidis, Hib, and S. pneumoniae were used in species-specific assays for ctrA, hpd, and lytA genes, respectively (Table 1) (18). The PCR assembly and cycling conditions were as follows: 5 µL of DNA, 1 µL of primers/probes, 12.5 µL of Invitrogen-Platinum Quantitative PCR SuperMix-UDG master mix, 1.5 µL of MgCl2 (50 nM), and water for a 25 µL final volume, run for 40 cycles at 95°C for 15 s and 60°C for one minute on a Rotor gene Q instrument (Kiagene Co.), as described previously. Positive results were verified by monoplex real-time PCR, and negative controls were included in all clinical samples' extraction processes. All negative samples were examined twice. A specimen was considered positive for all PCR assays if its CT value was ≤ 35 and negative if its CT value was > 40. If a CT value was > 35 and ≤ 40, the specimen was diluted 10 folds and retested (18).
| Target | Primer or Probe Name | Real-time Primer and Probe Nucleotide Sequence (5’to 3’) | Working Stock Conc. (µM) | Final Conc. (nM) | Suggested Probe Modifications |
|---|---|---|---|---|---|
| Neisseria meningitidis | |||||
| ctrA | F753 | TGTGTTCCGCTATACGCCATT | 3.75 | 300 | |
| R846 | GCCATATTCACACGATATACC | 11.25 | 900 | ||
| Pb20i | AACCTTGAGCAA”T”CCATTTATCCTGACGCGTTCT | 1.25 | 100 | 5’FAM,BHQ 1 on ”T”, 3’SpC6 | |
| sodC | F351 | GCACACTTAGGTGATTTACCTGCAT | 3.75 | 300 | |
| R478 | CCACCCGTGTGGATCATAATAGA | 7.5 | 600 | ||
| Pb896i | CATGATGGCACAGCAACAAATCCTGTTT | 1.25 | 100 | 5’ FAM,BHQ 1 | |
| Haemophilus influenza | |||||
| Hpd | HpdF822 | GGTTAAATATGCCGATGGTGTTG | 1.25 | 100 | |
| hpdR952 | TGCATCTTTACGCACGGT|GTA | 3.75 | 300 | ||
| Pb896i | 1.25 | 100 | 5’FAM,BHQ 1 on ”T”, 3’SpC6 | ||
| Streptococcus pneumonia | |||||
| lytA | F373 | ACGCAATCTAGCAGATGAAGCA | 2.5 | 200 | |
| R424 | TCGTGCGTTTTAATTCCAGCT | 2.5 | 200 | ||
| Pb400i | TGCCGAAAACGC”T”TGATACAGGGAG | 2.5 | 200 | 5’FAM,BHQ 1 on” T”, 3’SpC6 |
The viral RNA and DNA were extracted from 140 µL of each sample with a high pure viral nucleic acid kit containing proteinase K (Roche, Germany), according to the manufacturer’s instructions. Seven viruses, including non-polio human enteroviruses, mumps virus, Human Simplex virus (HSV), Varicella-Zoster virus (VZV), human cytomegalovirus (HCMV), human simplex virus type 6 (HHV-6), and Epstein-Barr virus (EBV), were tested using commercially purchased TaqMan real-time PCR assays from Genesig (Primerdesign, UK) on an ABI Step One Plus™ (Applied Biosystems, U.S.A) real-time PCR system according to the manufacturer’s instructions.
3.1. Statistical Analysis
Data were analyzed using the chi-square test, Fisher exact test, and independent t-test. The sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) were calculated for BM diagnosis based on the WHO criteria and the multiplex real-time PCR test results. A P value of < 0.05 was considered statistically significant. Statistical analysis was performed with SPSS Version 21.0.
4. Results
Totally, 78 out of 492 samples had more than five WBC counts per microliter in the CSF. Four patients with ventriculoperitoneal shunt were excluded from the study. Among 74 patients, there were 43 boys (58.1%) and girls (41.9%) with a mean age of 27 months.
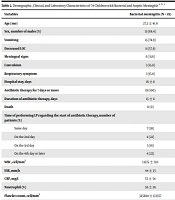
Nineteen patients had BM, including 17 patients diagnosed just by the multiplex real-time PCR for detection of S. pneumoniae (14 patients), Hib type b (two patients), and N. meningitidis (one patient), one by PCR for detection of Hib and positive Gram staining, and two by isolation of Salmonella typhi in CSF and/or blood cultures. We identified 55 patients with aseptic meningitis. Demographic, clinical, and laboratory characteristics of 74 children with bacterial and aseptic meningitis are shown in Table 2.
| Variables | Bacterial Meningitis (N = 19) | Aseptic Meningitis (N = 55) | P Value |
|---|---|---|---|
| Age (mo) | 27.2 ± 41.9 | 70.6 ±70.5 | 0.002 |
| Sex, number of males (%) | 13 (68.4) | 30 (54.4) | 0.291 |
| Vomiting | 15 (78.9) | 26 (47.3) | 0.015 |
| Decreased LOC | 11 (57.9) | 30 (54.4) | 0.800 |
| Meningeal signs | 6 (31.6) | 24 (43.6) | 0.405 |
| Convulsion | 3 (15.8) | 10 (18.2) | 0.560 |
| Respiratory symptom | 3 (15.8) | 4 (8.9) | 0.342 |
| Hospital stay, days | 16 ± 8 | 9 ± 6 | 0.003 |
| Antibiotic therapy for 7 days or more | 19 (100) | 35 (63) | 0.001 |
| Duration of antibiotic therapy, days | 15 ± 8 | 8 ± 6 | 0.002 |
| Death | 0 (0) | 0 (0) | - |
| Time of performing LP regarding the start of antibiotic therapy, number of patients (%) | < 0.001 | ||
| Same day | 7 (38) | 16 (30) | |
| On the 2nd day | 4 (22) | 10 (19) | |
| On the 3rd day | 3 (16) | 11 (21) | |
| On the 4th day or later | 4 (22) | 15 (19) | |
| WBC, cell/mm3 | 13575 ± 720 | 11178 ± 4813 | < 0.001 |
| ESR, mm/h | 44 ± 35 | 31 ± 20 | < 0.001 |
| CRP, mg/L | 55 ± 54 | 36 ± 47 | < 0.001 |
| Neutrophil (%) | 56 ± 26 | 51 ± 25 | < 0.001 |
| Platelet count, cell/mm3 | 325684 ± 133557 | 346458 ± 191501 | < 0.001 |
| CSF WBC, cell/mm3 | 541 ± 1411 | 11 ± 8 | < 0.001 |
| CSF neutrophil (%) | 30 ± 37 | 8 ± 16 | 0.024 |
| CSF protein, mg/dL | 138 ± 193 | 50 ± 27 | 0.063 |
| CSF glucose, mg/dL | 35 ± 17 | 60 ± 21 | < 0.001 |
Abbreviations: SD, standard deviation; N, number; LP, lumber puncture; LOC, level of consciousness; WBC, white blood cell; ESR, erythrocyte sedimentation rate; CRP, C-reactive protein; CSF, cerebrospinal fluid.
aValues are expressed as No. (%) and mean ± SD.
bLaboratory results are related to admission time or the first-time during hospital course.
cData were missed in four patients, one from the BM group and three from the aseptic meningitis group.
Based on the PCR test, 17 patients were diagnosed with BM, including 14 cases with S. pneumonia, two with Hib, and one with N. meningitides. Based on the WHO criteria, 17 patients had BM, including six by CSF leukocytosis more than 100, eight with leukocytosis between 10 and 100 and low glucose concentration or high protein concentration, one with positive Gram staining, and two with positive results of blood and/or CSF cultures. Demographic, clinical, and laboratory findings of BM children who were not diagnosed by the multiplex-PCR method or WHO criteria are shown in Table 3.
| No. | Age (m) | CP | PCR Test Result | Blood Culture | CSF Culture | CSF Analysis | TOL (Day) | HS (Day) | |||
|---|---|---|---|---|---|---|---|---|---|---|---|
| WBC (cell/mm3) | PMN (%) | Protein (mg/dL) | Glucose (mg/dL) | ||||||||
| 1 | 14 | F, V, C | N | Salmonella | Salmonella | 6100 | 95 | 240 | 3 | 2 | 40 |
| 2 | 1.5 | F, V | N | Salmonella | N | 15 | 90 | 100 | 202 | 1 | 21 |
| 3 | 15 | F, MS | N | N | 80 | 15 | 95 | 70 | 4 | 14 | |
| 4 | 24 | F | N | N | 5 | 10 | 10 | 58 | 4 | 14 | |
| 5 | 18 | F, MS | 8 | 2 | 45 | 52 | 5 | 10 | |||
Abbreviations: CP, clinical presentation; F, fever; V, vomiting; C, convulsion; MS, meningeal sign; N, negative; CSF, cerebrospinal fluid; P, protein; S, sugar; TOL, the timing of lumbar puncture; HS, hospital stay.
The sensitivities of the multiplex-PCR method and WHO criteria for diagnosing BM in our study were 89.4% and 84.2%, respectively, with specificities of 100% and 98.2%, positive predictive values of 100% and 94.1%, and negative predictive values of 96.5% and 94.1%, respectively (Tables 3 and 4).
| Groups | WHO Criteria Positive | WHO Criteria Negative |
|---|---|---|
| Confirmed bacterial meningitis | ||
| PCR positive | 14 | 3 |
| PCR negative | 2 | 0 |
| Aseptic meningitis | ||
| PCR positive | 0 | 0 |
| PCR negative | 1 | 54 |
Considering the time of performing LP relative to when commencing antibiotic therapy, we found that LP was requested on the day of starting empirical treatment for meningitis in all patients, but it was performed on the day of starting empirical treatment only in 23 patients (32.8%). In about half of the patients, LP was done on the third day of starting antibiotic therapy or later (Table 2).
All BM patients received antibiotics for a mean duration of 15 days (Table 2). Thirty-eight (69%) out of 55 patients with aseptic meningitis received antibiotic regimens for at least seven days. Thirty (86.8%) out of 38 patients received vancomycin in their antibiotic regimens.
Viral meningitis was diagnosed in 10 patients, including five enterovirus cases (6.75%), two HSV cases (2.7%), one CMV case (1.4%), one EBV case (1.4%), and one HHV-6 case (1.4%). No patients had VZV meningitis. According to the PCR result, one patient with a positive PCR result for HSV was a case of pneumococcal meningitis. This patient was a four-year-old girl who presented with fever and vomiting for five days. The CSF analysis reported 70 WBC/mm3, 100% lymphocytes, 40 mg/dL glucose, and 20 mg/dL protein. The CSF Gram staining and culture had negative results. She was treated with vancomycin and ceftriaxone for 10 days but did not receive acyclovir.
5. Discussion
Bacterial meningitis in children is an emerging infectious disease that can cause death or transient, persistent complications (19, 20). Rapid diagnosis and initiation of appropriate treatment can effectively reduce mortality and complications (21). Differentiation of BM from other causes of meningitis, especially viral meningitis, is crucial for the appropriate prescription of antibiotics in BM patients (22). In this study, 63% of the patients with aseptic meningitis received antibiotics for at least seven days. Overprescribing antibiotics could increase drugs' adverse effects, health care costs, and antibiotic resistance rate due to antibiotic selection pressure (23).
In this study, the sensitivity and specificity of WHO criteria for diagnosing BM and the multiplex RT-PCR for detecting common bacteria causing BM in children were high in a tertiary center in a developing country, consistent with previous ones (24). The PCR failed to detect only two BM cases caused by Salmonella enterica (Table 3). This pathogen is an uncommon cause of BM in infants and children and needs a prolonged course of antibiotic therapy (5, 6). Our patients aged 1.5 and 14 months received 21 and 40 days of antibiotic therapy, respectively. The WHO criteria in three patients without elevated white blood cells and protein levels did not detect BM. It seems to be due to delayed LP after starting antibiotic therapy. Besides, LP was performed at least four days after starting antibiotic therapy in these patients (Table 3). The significant effect of antibiotic administration before performing an LP on CSF profiles in BM children has been shown in previous studies. The study reported significant alterations in all CSF parameters associated with Hib meningitis detected after 48 hours of effective parenteral antibiotic therapy (25). In another study, an antibiotic administered within 72 hours before LP was associated with higher CSF glucose levels and lower CSF protein levels (26).
The PCR assay has a higher sensitivity and specificity than Gram staining and CSF and blood culture (21, 22). There are a few cases of BM confirmed by CSF culture in Iran and other developing countries. Some factors could be related to the low yield of CSF culture in these countries, including using antibiotics in febrile children before hospitalization, the delay in performing LP, the delay in transferring samples from the department to the laboratory, and inappropriate laboratory procedures for the isolation of meningitis pathogens. The use of antibiotics in febrile children before hospitalization is common in developing countries. In a nationwide study in Iran, antibiotics were utilized by 62.6% of children with acute respiratory tract infection (27). We observed a delay in performing LP in our center. Parents' refusal to give consent to LP for their children is a common cause of delay in performing LP in children suspected of meningitis in our center. The same problem was reported from other centers, especially in the Middle East (28, 29). The main reasons are the lacked knowledge and a misconception that LP is a harmful procedure that will result in the paralysis of the child, infertility, especially in the male gender, urine incontinence, and/or the aggravated course of the disease. The delay in transferring samples from the department to the laboratory could be another cause of the low yield of CSF culture in BM patients in our center. According to the recommendation of the Centers for Disease Control and Prevention (CDC), CSF should be processed in a microbiology laboratory within one hour after collection or inoculated into the Trans-Isolate (T-I) medium before transport to the laboratory if processing within one hour is not feasible (29). Inappropriate laboratory procedures for the isolation of meningitis pathogens may be the other cause.
In this study, enterovirus was the most common viral etiology in children with meningitis, consistent with other studies and a previous study in our center (30, 31). A patient with pneumococcal BM had a positive PCR test result for HSV in the CSF. Coinfection of enteroviral meningitis and BM has been reported (32, 33). Research has shown that the association of either herpes virus, CNS, or EBV with bacterial meningitis in adult patients increases mortality. Most of these individuals had been infected with the human immunodeficiency virus (34). Our patient was a four-year-old girl treated empirically with vancomycin and ceftriaxone, with complete improvement.
This study has some limitations. This is a single-center study performed retrospectively. Otherwise, the number of patients was sufficient to evaluate the accuracy of the diagnostic methods in our center.
In conclusion, the WHO criteria and multiplex real-time PCR had high accuracy in diagnosing the three most common causes of childhood BM. Performing LP before starting antibiotics could increase the sensitivity of WHO criteria. The presence of uncommon etiology of BM in children was a significant limitation of the PCR to detect common bacterial etiologies of BM. Using the WHO criteria, the multiplex real-time PCR test, and blood and CSF cultures to decide on stopping or changing antibiotics prescribed empirically in febrile children suspected of BM could help avoid the antibiotics overprescription while avoiding mortality and morbidity of BM.